Case 3 Revisited: Finding a diagnostic approach – Part 2
Invasive aspergillosis is a challenging condition to diagnose, treat and manage. Mortality is high, even when the diagnosis is clear and treatment is started early.
Much like we saw in Case 2, over-cautious clinicians may wish to use empirical and prophylactic antifungals in a large number of patients. The use of antifungals like voriconazole require specialist input – they can be difficult to use and have significant adverse effects.
The goal of the Antifungal Stewardship team in this setting should be to develop guidelines which can help clinical teams:
- to use empirical antifungals only in patients who are most likely to benefit from them
- to choose antifungals which are most likely to be effective in various patient scenarios
You may have suggested improvements to this patient’s treatment already in the previous Discussion page.
Firstly, we will review the stewardship principles that apply to Mrs Bell’s management. This will explore the important considerations for IA treatment, which can also apply to other invasive fungal infections. Then we can look at an example of a successful stewardship project which has helped clinicians identify the right time to use empirical antifungals in the setting of neutropenia.
Right Drug, Right Time, Right Dose, Right Route and Right Duration
The principle of giving “the right drug, at the right time and dose, via the right route and for the right duration” applies directly to Barbara Bell’s management. Read them and reflect on whether you had identified these issues whilst looking into Case 3.
Right Drug – Multiple factors influence our choice of antifungals here. Did you consider all of them?
- Antifungal prophylaxis: many haematology patients like Barbara are prescribed mould active antifungal prophylaxis (posaconazole) as they are at risk of IA. Her medication history contained “antimicrobial prophylaxis” which probably included this. Patients who develop a “breakthrough” fungal infection on mould active azole prophylaxis should be treated with an antifungal from an alternative class – namely liposomal amphotericin B – as first line.
- Antifungal resistance: fungal culture obtained at bronchoscopy should always be sent for antifungal susceptibility testing. Barbara’s bronchoscopy samples went on to grow an Aspergillus fumigatus resistant to voriconazole, posaconazole and isavuconazole. This information stresses the importance of timely diagnostic tests (read more in the Right Time section).
- Combination therapy: what treats the invasive aspergillosis for the first few days before voriconazole reaches therapeutic levels in the body? In critically unwell patients, combining voriconazole with liposomal amphotericin B for a few days (until target drug level is achieved or susceptibility results are available) is often recommended, particularly in cases with risk for azole resistance.
- For more information on antifungal choice, you can click here for international guidance on treatment of invasive aspergillosis. Have a close look at the recommended indications for antifungal prophylaxis.
Right Time – How do you think this could have been improved for Barbara?
- Empirical antifungals were started as soon as IA was considered a possibility – this is usually appropriate.
- What about the timing of essential diagnostics? Serum fungal biomarkers were sent in a timely fashion, but her bronchoscopy was delayed by 48 hours. Samples taken on antifungal treatment are less sensitive, impairing the diagnosis. A delay in sampling also delays antifungal resistance being detected and effective treatment started.
Right Dose – Barbara did not improve on antifungal treatment. Did she get the right dose?
- Therapeutic drug monitoring is essential when using voriconazole and other triazoles. Had the medical team taken appropriate voriconazole levels (trough levels on 3rd day of treatment), they would have recognized sub-therapeutic dosing. This could have explained her lack of improvement even in the absence of antifungal resistance.
- You can review guidance for monitoring azole antifungals by clicking this link.
Right Route – Did you consider the most appropriate route for Barbara’s treatment?
- Given her deteriorating clinical condition, starting her on intravenous antifungals was likely the right choice.
- Had she improved on the right treatment, switching her to an appropriate oral treatment would be the next step. But please note there are no oral non-azole alternatives for azole resistant Aspergillus infections.
Right Duration – Did you feel confident estimating the necessary treatment duration for Barbara?
- Early in treating invasive aspergillosis, it would be reasonable to be uncertain about duration of treatment.
- However, antifungals should be regularly reviewed for their appropriateness. Barbara was given 4 days of intravenous voriconazole before it was reviewed. Could earlier review have identified some of the issues described?
Finding a diagnostic approach
The clinical team suspected Barbara to have developed invasive aspergillosis due to her risk factors, clinical picture and CT scan findings. It was then confirmed on fungal biomarker testing and eventually by culture of the bronchoscopy samples.
In order to avoid the unnecessary use of empirical antifungals, a robust guideline is needed for the clinical team to follow. In the absence of this, the high mortality and non-specific nature of IA will lure the clinician into overuse of antifungals.
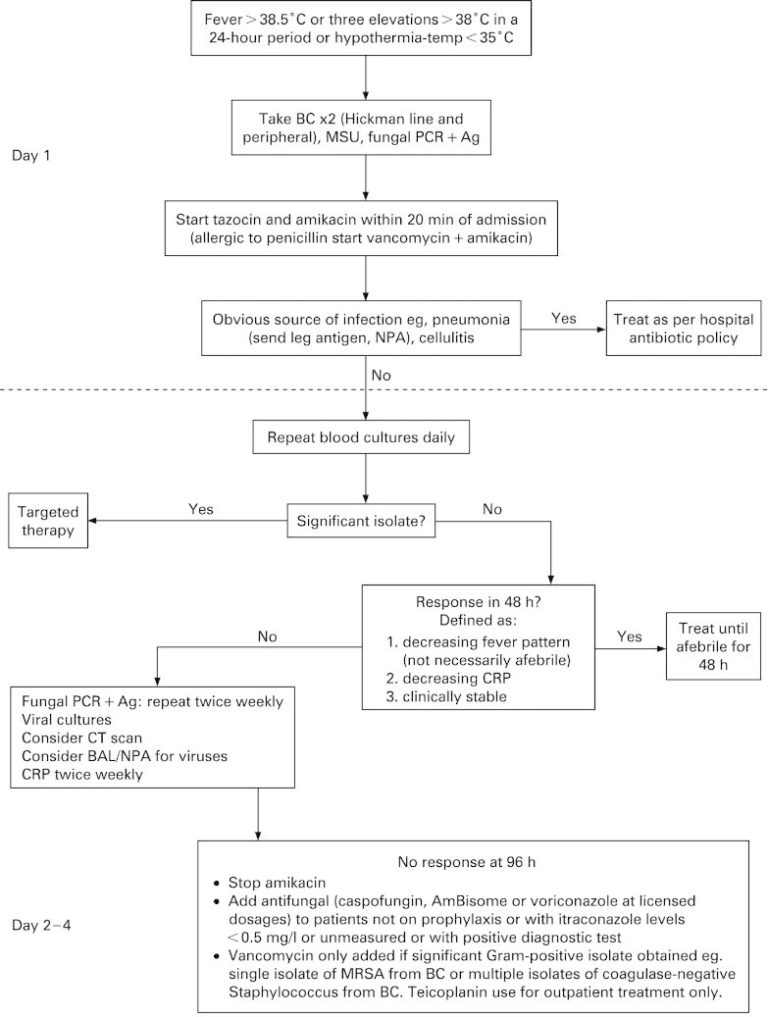
One effective and safe approach to antifungal stewardship in patients at risk for IA is summarised in the below image. In high-risk haematology patients, fever triggers prompt fungal biomarker testing. Clear guidance on timing of treatment is offered and liked with timely fungal biomarker tests. More common alternative causes (such as bacteria) are always considered. Enforcing this policy of restricted antifungal use was shown not to impact mortality associated with invasive fungal infections, but did reduce antifungal use. You can read more about this stewardship project by clicking this link.
It is the task of the Antifungal Stewardship team to develop such guidance locally. This should be done in collaboration with the clinical teams, with key clinical areas having a representative in the AFS team. Guidelines need to be specific to the patients seen in the hospital/department and therefore are likely to differ slightly from generic guidelines. Local resistance patterns and incidence of disease need to be taken into consideration when developing the guidelines. Knowledge of the local patient population is thus important.
The Stewardship team’s input does not end to providing these tools. It is crucial that the efficacy and safety of the guideline is audited regularly. Also, education and support needs to be provided to the clinical staff and maintaining a multidisciplinary approach to monitoring adherence is essential.
Can you think of any barriers to implementing a guideline like this? You may be able to think of your own approach to stewardship in this area. Feel free to share it in the comment section below.
Share this

Reach your personal and professional goals
Unlock access to hundreds of expert online courses and degrees from top universities and educators to gain accredited qualifications and professional CV-building certificates.
Join over 18 million learners to launch, switch or build upon your career, all at your own pace, across a wide range of topic areas.
Register to receive updates
-
Create an account to receive our newsletter, course recommendations and promotions.
Register for free