Home / Science, Engineering & Maths / Biology & Biotechnology / Biochemistry: the Molecules of Life / Definitions of common biochemical terms

Reach your personal and professional goals
Unlock access to hundreds of expert online courses and degrees from top universities and educators to gain accredited qualifications and professional CV-building certificates.
Join over 18 million learners to launch, switch or build upon your career, all at your own pace, across a wide range of topic areas.

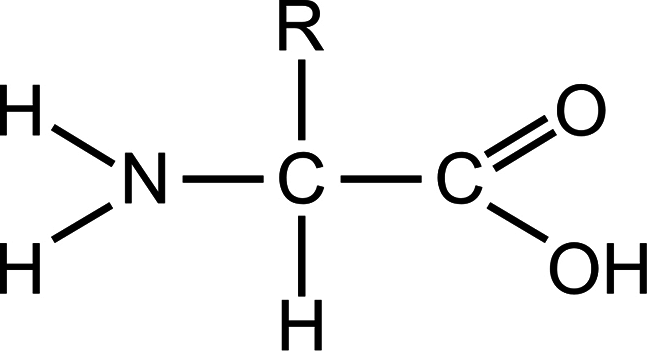 Chemical structure of a standard amino acid in its unionised form. “R” represents a group of atoms that varies for each specific type of amino acid.
Chemical structure of a standard amino acid in its unionised form. “R” represents a group of atoms that varies for each specific type of amino acid.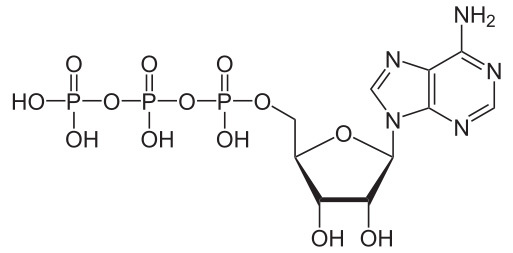 Chemical structure of adenosine 5’-triphosphate.
Chemical structure of adenosine 5’-triphosphate.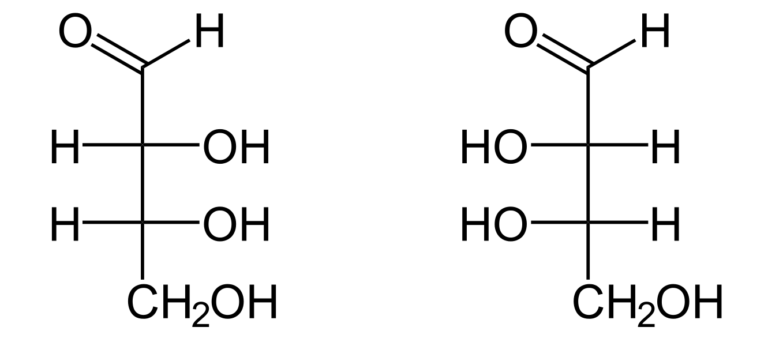 Chemical structures of two stereoisomers of erythrose, a 4-carbon monosaccharide. The structures show D-erythrose (left) and L-erythrose (right).
Chemical structures of two stereoisomers of erythrose, a 4-carbon monosaccharide. The structures show D-erythrose (left) and L-erythrose (right).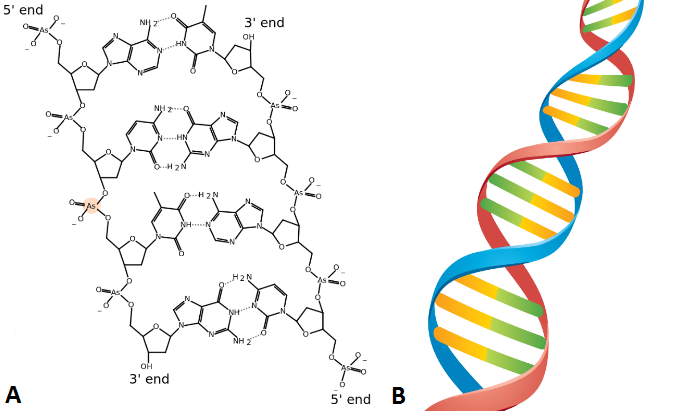 Representations of the structure of deoxyribonucleic acid (DNA). (A) Chemical structure of DNA, highlighting the base pairs that connect the two strands. (B) Molecular structure of DNA, highlighting how the two strands coil around each other to form a double helix. Source: bio.libretexts.org CC BY-NC-SA 3.0
Representations of the structure of deoxyribonucleic acid (DNA). (A) Chemical structure of DNA, highlighting the base pairs that connect the two strands. (B) Molecular structure of DNA, highlighting how the two strands coil around each other to form a double helix. Source: bio.libretexts.org CC BY-NC-SA 3.0 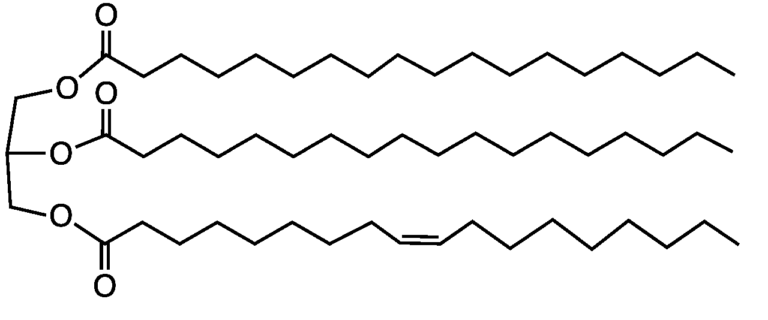 Chemical structure of lipid, showing the hydrophilic end to the left of the diagram.
Chemical structure of lipid, showing the hydrophilic end to the left of the diagram.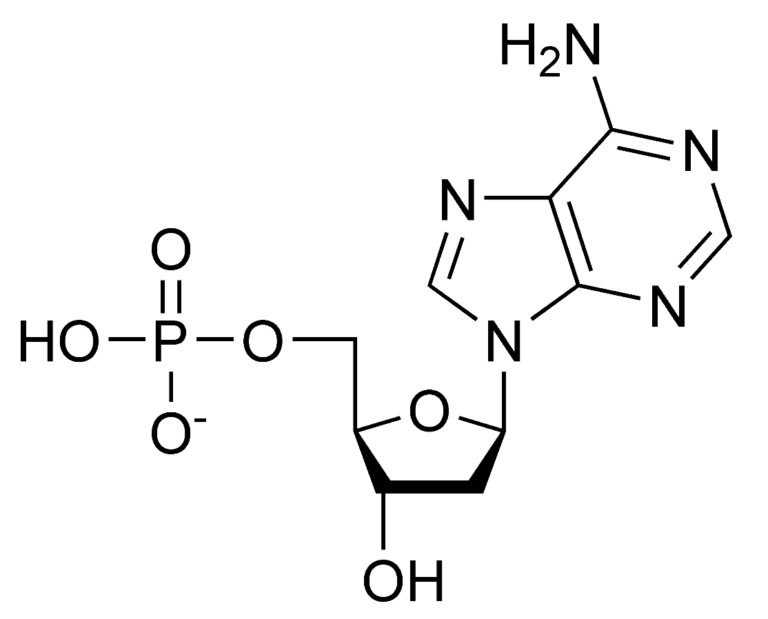 Chemical structure of nucleotide monomer.
Chemical structure of nucleotide monomer.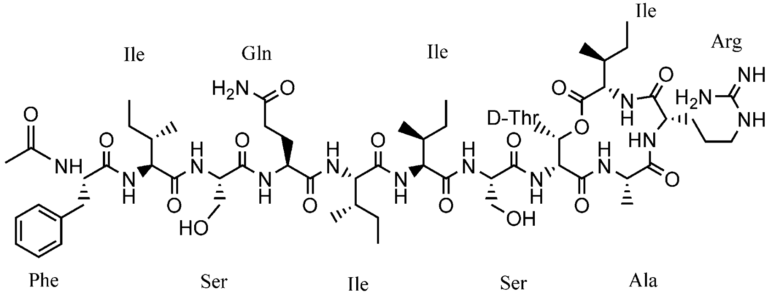 Chemical structures of polypeptide containing 10 amino acids, each indicated their 3-letter abbreviation.
Chemical structures of polypeptide containing 10 amino acids, each indicated their 3-letter abbreviation.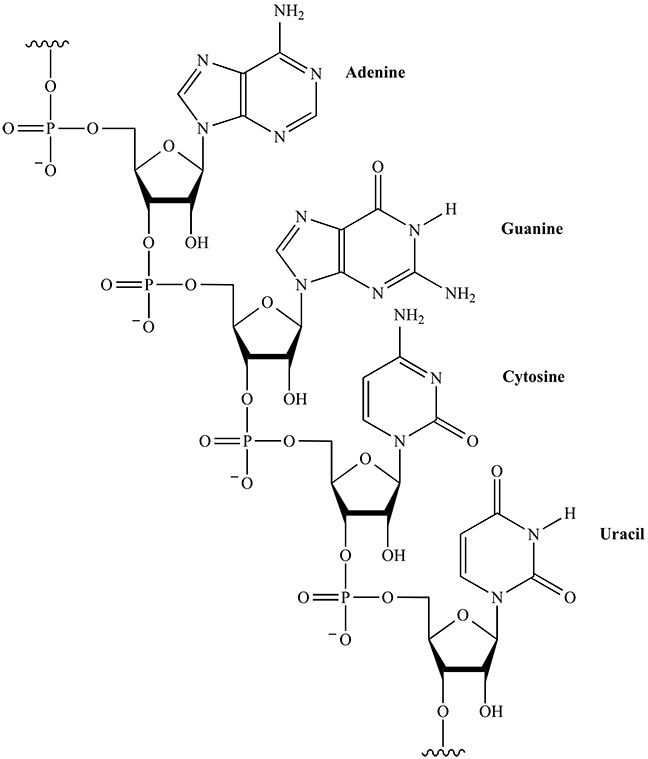 Chemical structure of single chain RNA, highlighting that each ribose contains a free hydroxyl group (OH).
Chemical structure of single chain RNA, highlighting that each ribose contains a free hydroxyl group (OH).






