Technical Words in Every Day Chemistry – a Glossary

Share this step
A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
A
ACETAL
An acetal is a functional group with the following structure R2C(OR)2 or RCH(OR)2. It is formed by the condensation of two alcohol (ROH) molecules with an aldehyde (RCHO) or ketone (RCOR). (In comparison, a hemiacetal has the structure R2C(OR)(OH) or RCH(OR)(OH), and is formed by the condensation of one alcohol molecule with an aldehyde or ketone.)
ACETONE
Acetone (or propanone) is the simplest ketone, with the formula CH3COCH3. It is a colourless, low-boiling, flammable liquid that is an important solvent, including for cleaning purposes in the chemistry laboratory. Acetone is present in nail polish remover and paint thinner.
ACIDITY
The tendency of a compound to act as a proton or hydrogen ion (H+) donor. Common aqueous acids include hydrochloric acid (HCl), acetic acid (or ethanoic acid, CH3CO2H) and sulfuric acid (H2SO4). Loss of a proton from an acid of general formula HA, forms the anion A–. The more stable A– is, the stronger the acid HA. For example, carboxylic acids (RCO2H) are stronger acids than alcohols (ROH), because the carboxylate ion (RCO2–) is more stable than the alkoxide ion (RO–). (The carboxylate ion is stabilised by resonance.)
ACTIVE SITE
The active site is the region of an enzyme where a substrate binds and undergoes a chemical reaction to form a product (or products). Groups within the active site make temporary bonds (e.g. hydrogen bonds) to the substrate and the active site is often a groove or pocket within the enzyme. An active site can catalyse a reaction time and time again, as the groups that makes bonds to the substrate are not altered at the end of the reaction.
AGAR
A mixture derived from the polysaccharide agrose which is used as a growth medium for growing microbes in the laboratory. Microorganisms placed on the plate will grow into individual microbial colonies.
ALCOHOL
A compound in which a hydroxyl group (–OH) is bonded to a saturated carbon atom. For example, methanol (CH3OH) and ethanol (CH3CH2OH) are alcohols. Primary alcohols have the general formula RCH2OH, while secondary have the formula R2CHOH and tertiary have the formula R3COH. Most of the common alcohols are colourless liquids at room temperature and ethanol, for example, is the alcohol in alcoholic beverages. Ethanol and isopropanol (2-propanol, CH3CH(OH)CH3) are amongst the most widely-used antiseptics.
ALDEHYDE
An aldehyde is the name for a group of organic compounds that contain a carbonyl group (C=O) that is bonded to a hydrocarbon group (–CnH2n+1) and a hydrogen atom (RCHO). The C=O bond is located at the end of the carbon chain; for example, in butanal (shown below).
ALKENE
A series of unsaturated hydrocarbons containing a C=C double bond. The number of hydrogen atoms in an alkene is double the number of carbon atoms. For example, the molecular formula of ethene is C2H4 (H2C=CH2), while for propene it is C3H6 (H2C=CH–CH3). Rotation about the C=C double bond is restricted so substituted alkenes may exist as one of two isomers, called cis- or trans-, or Z– or E– isomers.
ALKYL GROUP
Formed on loss of a hydrogen atom from an alkane. For example, loss of H from ethane, CH3CH3, gives an alkyl group called an ethyl group, CH3CH2–. The symbol R in a structure indicates an alkyl group.
AMIDE
An amide functional group has the structure R–(C=O)–NR2 (tertiary amide), R–(C=O)–NHR (secondary amide) or R–(C=O)–NH2 (primary amide). This functional group is found in protein chains – it links amino acids together. The amine group of one amino acid reacts with the carboxylic acid group of another amino acid, to form the amide bond, in a condensation reaction. The amide below is called N-ethylpropanamide.
AMINE
A compound in which a hydrogen atom, or atoms, in ammonia is replaced by alkyl or aryl groups. For example, methylamine (CH3NH2) and ethylamine (CH3CH2NH2) are amines. Primary amines have the general formula RNH2, while secondary have the formula R2NH and tertiary have the formula R3N. A representative tertiary amine is triethylamine Et3N (where Et stands for ethyl, CH3CH2–).
AMINO ACIDS
The building blocks of all proteins. There are 20 found in the universal genetic code, of which 9 are classed as essential and must be taken up as food as they cannot be directly synthesised by the human body. All amino acids share the same basic structure of an amine group and carboxylic acid group bonded to a central carbon atom (called the alpha carbon). This carbon atom is also bonded to a hydrogen atom and a variable R group. The simplest amino acid, glycine, contains another hydrogen atom at this variable point, H2N–CH2–CO2H.
ANALOGUE
A molecule that shares the same basic structure (backbone) as its parent compound; however, variable sites contain different functional groups. Despite being so similar, structural analogues can have very different physical, chemical or biological properties.
ANTIBIOTIC
A compound that interferes with the growth of bacteria. It may either kill or inhibit the growth of bacteria.
ANTIMICROBIAL
A compound that kills microorganisms (bacteria, viruses, fungi and protozoa) or inhibits their growth. Antimicrobial medicines are grouped according to the microorganisms they act primarily against. For example, antibiotics are used against bacteria, antivirals are used to treat viral infections, and antifungals are used against fungi.
ANTIOXIDANT
A compound, such as vitamin C or E, that removes potentially damaging oxidising agents in a living organism. They reduce damage due to oxygen, such as that caused by free radicals.
ARYL GROUP
A functional group or substituent that is an aromatic ring e.g. a phenyl group (–C6H5) or a substituted phenyl group.
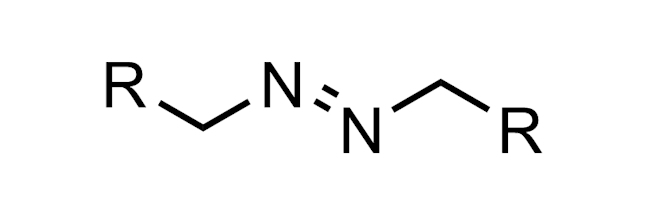
AZO GROUP
A functional group with the structure R–N=N–R. This group is present in early azo dye antibiotics such as Prontosil.
B
BACON
Cooking bacon produces 150 volatile organic compounds. On heating, fats in the bacon breakdown, and sugars react with amino acids (in the Maillard reaction), to produce a mixture of hydrocarbons, aldehydes, ketones, alcohols and nitrogen-containing compounds that are responsible for the aroma of frying bacon. Interestingly, the chef Heston Blumenthal and experimental psychologist Charles Spence demonstrated that people rate bacon-and-egg ice-cream as tasting significantly more bacony when listening to the sound of sizzling bacon. So, an accompanying soundtrack can modify the way food tastes!
BACTERICIDAL
A molecule or compound which actively kills bacterial cells.
BACTERIOSTATIC
A molecule which does not kill bacterial cells but inhibits their growth and multiplication.
BACTERIUM
A type of microorganism just a few micro-metres in length. It is a prokaryotic cell, categorised by the absence of a membrane-bound nucleus.
BASICITY
The tendency of a molecule to share a pair of electrons with a proton (forming a new bond to hydrogen). The more available the electrons, the more readily they can be donated to form a new bond to the proton. For example, ammonia (H3N:) is a stronger base than water (H2O:) because the lone pair of electrons on nitrogen are more available than the lone pairs of electrons on oxygen (nitrogen is less electronegative than oxygen).
BENZENE
Benzene has the molecular formula C6H6. Kekulé was the first to suggest a sensible structure of benzene where the carbons are arranged in a hexagon, and he suggested alternating double and single bonds. This is not correct because, for example, all the carbon-carbon bonds are exactly the same length – the electrons in the double bonds are delocalised (or spread) around the ring, which is represented by drawing a circle inside the hexagon. However, the Kekulé structure can be useful when drawing reaction mechanisms, or for showing delocalisation, using curly arrows.
BETA-LACTAM RING
A four-membered cyclic amide (lactam) in which the nitrogen atom is placed at the β-position relative to the carbonyl group. (The carbon atom next to a C=O group is called the alpha carbon and a carbon atom joined to this is called the beta carbon.)
BIOTECHNOLOGY
A broad discipline in which biological processes, organisms, cells or cellular components are exploited to develop novel technologies. New tools and products developed by biotechnologists are useful in research, agriculture, industry and the clinic. For example, biotechnology will have a huge impact on the flavours and fragrances industry. For example, companies are focusing efforts on using microorganisms to produce nootkatone, which has an unmistakeable citrus odour. The chemical can be extracted directly from grapefruits or from certain cedar trees. Alternatively, it can be produced by oxidising another flavour ingredient, called valencene, which is found in oranges.
BOND ANGLE
The angle that is formed between two adjacent bonds on the same atom.
C
CAFFEINE
Caffeine is a bitter substance found in coffee, tea, soft drinks, chocolate, kola nuts, and certain medicines. It is a central nervous system (CNS) stimulant and the world’s most widely consumed pyschoactive drug. It works by reversibly blocking the action of adenosine on its receptor, which consequently prevents the onset of drowsiness induced by adenosine. You may have read about a recent incident where two students were nearly killed after they were given enough caffeine for 300 cups of coffee during a ‘botched’ experiment – short-term memory loss and loss of weight was observed – thankfully, both students made a full physical recovery.
CARBAMATE
A functional group with the structure R–O–(C=O)–NR2 or R–O–(C=O)–NHR, sometimes called a urethane.
CARBOHYDRATE
A group of organic compounds (containing carbon, hydrogen and oxygen atoms) that includes sugars, starches, celluloses, and gums and serves as a major energy source in the diet of animals. They are polyhydroxy aldehydes and ketones (i.e. they contain one –CHO or –COR group, and many OH groups) and have various roles in living organisms including storage of energy (e.g. starch) and as structural components (e.g. cellulose in plants).
CARBONATE
An organic carbonate is a functional group with the structure R–O–(C=O)–O–R.
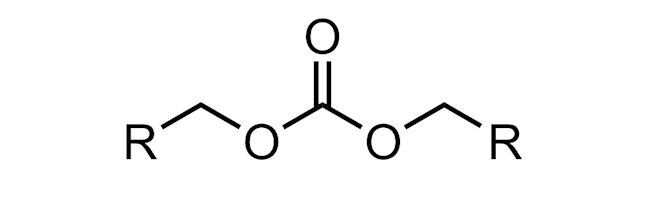
Monomers of polycarbonates are linked by carbonate groups.
CARBOXYLIC ACID
An organic compound containing a carboxyl group (COOH) with the general formula RCOOH (or RCO2H). They are proton donors and loss of a proton forms a carboxylate ion, RCO2–, which is stabilised by resonance. A common carboxylic acid is ethanoic acid, or acetic acid (vinegar), which has the formula CH3CO2H.
CATALYST
A species that increases the rate at which a reaction occurs without being consumed in the reaction. It is chemically unchanged at the end of the reaction it has been used to speed up, or catalyse, and works by providing an alternative route for the reaction. Consequently, only tiny amounts are typically required.
CELL MEMBRANE
The cell membrane is a biological membrane that separates the interior of all cells from the outside environment. It consists of a lipid bilayer (each lipid molecule contains a hydrophilic, or polar head region, and a hydrophobic, or nonpolar tail region) with embedded proteins. The hydrophobic lipid ends face inward and the hydrophilic ends face outward.
CELLULOSE
An insoluble substance which is the main constituent of plant cell walls and of vegetable fibres such as cotton. It is a polysaccharide consisting of chains of glucose monomers.
CHIRAL CENTRE
Chiral centres are tetrahedral atoms (usually carbon) which have four different groups attached to them. The (+) and (-) labelling of enantiomers relates to rotation of a special form of light by the enantiomer – when a compound rotates the light clockwise it is called the (+)-enantiomer, whereas if the enantiomer rotates the light anticlockwise it is called the (-)-enantiomer. An alternative (more useful) system to the (+) and (-) labelling is the CIP system, which uses the terms R and S to indicate the arrangement of the groups around the chiral carbon. The four groups attached to the chiral carbon are prioritised using a set of rules – if the priorities of the groups follow a clockwise direction, the chiral centre is assigned to be R (and S for an anticlockwise direction).
CINNAMALDEHYDE
The molecule responsible for giving cinnamon its flavour and odour. In nature, the C=C bond is mainly the trans– or E-isomer.
CIS and TRANS
For an alkene of type RCH=CHR, if the two alkyl groups (R) are on the same side of the C=C bond the alkene is assigned as the cis-isomer. If the two alkyl groups (R) are on the opposite sides of the C=C bond, the alkene is assigned as the trans-isomer. These isomers are called stereoisomers – compounds with the same structural formula but with a different arrangement of their atoms in space.
CITRIC ACID
A tricarboxylic acid found naturally in citrus fruits (e.g. lemons and limes), with the formula C6H8O7 (HO2CCH2C(OH)(CO2H)CH2CO2H). It is widely used as a flavouring agent.
COLLOID
A colloid is a material in which two materials that normally don’t mix are forcibly mixed – small particles of one substance (gas, liquid or solid – the disperse phase) are suspended (or floating) in another (the continuous phase – gas, liquid or solid). All combinations, except gas in gas, are possible and we meet colloids frequently in our everyday lives. Black filter coffee, for example, is a colloid of coffee solids in water with particles between 1 nm and 1000 nm (so small that they pass through the pores of filter paper). If you dilute your coffee by adding plenty of water it will be apparent that this is a genuine solution, as it is no longer cloudy, but clear. The foam on top of a cappuccino is another colloid – tiny bubbles of air (the disperse phase), are suspended in the continuous phase of milk (which itself is a colloid). Coffee-making machines produce yet another colloid, this time an aerosol: Tiny droplets of the disperse phase (water condensed from steam as it cools) floating in air (the continuous phase).
CONDENSATION REACTION
A chemical reaction involving two reactants in which a small molecule, typically water, is formed as a by-product.
CONFIGURATION
The 3-dimensional arrangement of atoms or groups in a molecule to give configurational isomers that cannot be interconverted without breaking a bond e.g. cis– and trans– alkenes are configurational isomers, as are R and S enantiomers.
CONFORMATION
The 3-D arrangement of atoms that results from rotation of a single bond. A specific conformation of a molecule that is relatively stable is called a conformer, or conformational isomer. For example, the most stable conformation of butane (CH3CH2CH2CH3) has the four carbon atoms arranged in a zigzag shape.
CONTROL SAMPLE
The control sample in an experiment is the sample which does not receive any treatment and is used as a benchmark against which other test results are measured.
COVALENT BOND
This is a bond that results from the sharing of an electron pair between two atoms to make a chemical bond. In a covalent bond, one electron comes from each atom involved in the bonding, e.g. for a C–H covalent bond, one electron will come from carbon and one from hydrogen.
CROSS–LINKING
A chemical bond between different chains of atoms in a polymer or other complex molecule.
CURLY ARROWS (DOUBLE HEADED)
Using a double-headed curly arrow in a mechanism indicates the movement of an electron pair. The end of the arrow represents the electron-pair source and the head of the arrow indicates the electron-pair destination.
CURLY ARROWS (SINGLE HEADED)
The presence of a single-headed curly arrow in a radical mechanism indicates the movement of a single electron.
D
DAMPING
Damping is the energy dissipation properties of a material or system that is under stress. For example, the shock absorbers on a car critically damp the suspension of the vehicle and so resist the setting up of vibration, which could make control difficult or cause damage.
DEHYDRATION
Loss of water. In a dehydration reaction, the reaction leads to the loss of a molecule of water. For example, when a carboxylic acid (RCO2H) is treated with an alcohol (ROH) and an acid catalyst, an ester (RCO2R) is formed (along with water).
DELOCALISATION
When lone pairs, electrons in double or triple bonds, or unpaired electrons, are spread over several atoms. Electrons that are shared by more than two atoms are called delocalised electrons. A molecule or ion with delocalised electrons is represented by resonance structures interconnected by double-headed arrows called resonance arrows, <–>. The resonance structures are used collectively to describe the actual molecular structure, which is an approximate intermediate between the resonance structures, called a resonance hybrid.
DENATURED
Denaturing is the process by which the characteristic properties of a biological macromolecule (including proteins and enzymes) are destroyed. Denaturing can be the result of applying heat, acidic or alkali conditions or another effect that can result in a change to the conformation of the macromolecule.
DEPROTONATE
To remove a proton (H+).
DEUTERATED
A compound is deuterated when one or more hydrogen atoms are replaced with deuterium. Deuterium (D or 2H), sometimes called heavy hydrogen, is an isotope of hydrogen with a nucleus consisting of one proton and one neutron. For example, deuterated propanone (or acetone, H3C–CO–CH3) has the formula D3C–CO–CD3.
DIASTEREOMERS
Diastereomers (or diastereoisomers) are stereoisomers of a molecule that differ at one or more chiral centres, but are not mirror images and are, therefore, not enantiomers. For example, the two compounds below are diastereomers.

They are not mirror images of each other, but are still the same compound while having a different arrangement of groups at one chiral centre (and the same arrangement of groups at the other chiral centre).
DIENE
A hydrocarbon that contains two carbon-carbon double bonds, C=C. In a conjugated diene, the two double bonds are separated by one single bond e.g. 1,3-butadiene, H2C=CH–CH=CH2. Unconjugated dienes have the double bonds separated by two or more single bonds.
DNA
DNA, or deoxyribonucleic acid, contains the instructions an organism needs to develop, live and reproduce. It consists of two strands of building blocks (or monomers), called nucleotides, that are linked together in a structure resembling a ladder twisted into a spiral (called a double helix).
DOT AND CROSS DIAGRAM
A dot and cross diagram indicates the electrons from both atoms that go into making the bond. The full structural formula shows all the bonds to all the atoms in the molecule.
DUCTILITY
Ductility is a measure of a solid material’s ability to withstand tensile stress, which is a force pulling the two ends of a material away from each other. If ductile, a material may be stretched into a wire.
E
E AND Z CONFIGURATION
A systematic approach to assign the stereochemistry of any substituted alkene. Atoms or groups attached to the C=C bond are given priorities, using a set of sequence rules. If the groups of higher priority are on the same side of the C=C bond, the alkene has the Z-configuration (like for cis-alkenes). If the groups of higher priority are on the opposite sides of the C=C bond, the alkene has the E-configuration (like for trans-alkenes). These isomers are called stereoisomers – compounds with the same structural formula but with a different arrangement of their atoms in space.
ELECTRON-DONATING GROUP
An atom or group that releases electron density from neighbouring atoms. Alkyl groups (such as methyl, CH3–, and ethyl, CH3CH2–) are electron-donating groups.
ELECTRON-WITHDRAWING GROUP
An atom or group that draws electron density from neighbouring atoms. Halogen atoms are electron-withdrawing groups.
ELECTRONEGATIVE
An atom that attracts electrons towards itself. Atoms with high electronegativity form negative ions (anions) and, when covalently bonded to atoms of lower electronegativity, have a greater share of the electrons than the other atom. Fluorine is the most electronegative element, followed by oxygen, chlorine and nitrogen.
ELECTROPHILE (LEWIS ACID)
An electrophile is a neutral or positively charged species which can accept a pair of electrons. It participates in a chemical reaction by accepting an electron pair to form a bond to a nucleophile. For example, the simplest electrophile is H+ (a proton).
ENANTIOMERS
Enantiomers are molecules that are non-superimposable mirror images of each other. Therefore, no matter how you rotate the two enantiomers, you will never be able to get them to look exactly the same. If you imagine two snails which are mirror images of each other, like in the picture above. Snail shells spiral out in a cone like shape; therefore, a snail does not look the same from both sides. On the side facing towards us we have the spiral shell, on the side facing away from us is just smooth shell. So if we turned snail 2 to face the same way as snail 1, the following would happen.
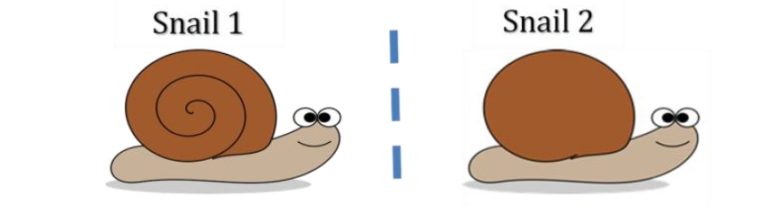
Therefore, when we rotate snail 2 to try and look like snail one, we see the back of the shell instead of the front. No matter how we rotate snail 2, we will never be able to make snail 2 look the same direction and have the shell facing and spiralling the same way. Now, we can relate the snail to organic compounds in the following way. The following image shows two enantiomers of the fragrance molecule citronellol.
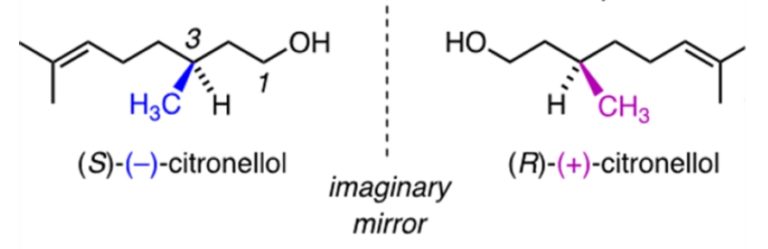
Now, we are going to imagine the wedged –CH3 group (which points forwards) as the swirl on the snails pointing forwards, and the hashed –H (pointing backwards) as the smooth shell on the back of the snail. So if we rotate the molecules so H is wedged we see the back of the snail.
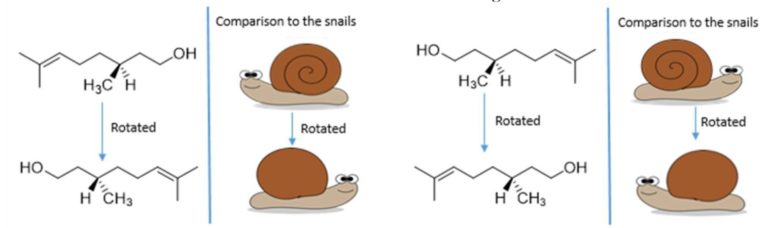
As you can see, by rotating (R)-(+)-citronellol and (S)-(-)-citronellol to get the two molecules facing the same direction to each other with the groups in the right places (just like we did to the snails), that there is no way that we can rotate (R)-(+)-citronellol and make it look exactly the same as (S)-(-)-citronellol, and vice versa, because the –CH3 and the –H groups will always be pointing in the wrong direction. Just like with the snails. Enantiomers are also like golf clubs or shoes – as most people have a particular handedness they require different golf clubs, as both golf clubs and our hands are chiral. A right-handed person requires a right-handed golf club, rather than a left-handed golf club, to be able to hit the ball.
ENZYME
An enzyme is a catalyst made up of multiple protein chains. The enzyme has an active site which binds to a specific substrate with a complimentary binding site to that of the enzyme active site. The enzyme converts the substrate into a product(s), but remains chemically unchanged in the process; therefore, acting as a catalyst.
EPOXIDE
A 3-membered cyclic ether (R–O–R). The ring comprises an oxygen atom and two carbon atoms. The simplest epoxide, with the formula C2H4O, is called ethylene oxide (or oxirane).
EPOXY RESIN
A substance formed by mixing a low-molecular-weight ‘prepolymer’ (a substance that represents an intermediate stage in polymerisation) with a compound that forms a cross-linked polymer.
ESTER
A functional group with the structure R-(C=O)-O-R. Simple alkyl esters can have pleasant, fruity smells. The ester below is called ethyl propanoate.
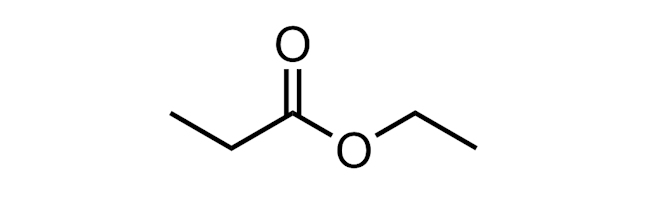
Esters are formed in esterification reactions, for example, on reaction of a carboxylic acid (RCO2H) with an alcohol (ROH) in the presence of an acid catalyst.
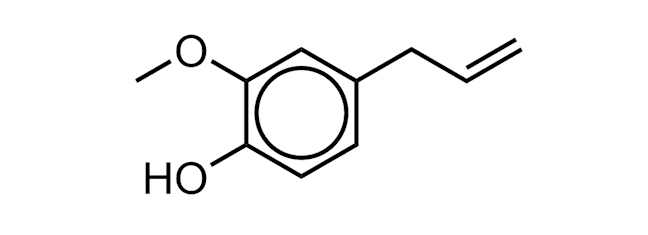
EUGENOL
A molecule found in essential oils of basil, cloves, nutmeg and cinnamon. It has a variety of uses in perfumes and flavourings.
F
FATIGUE
The weakening of a material caused by repeatedly applied loads. It is the progressive and localised structural damage that occurs when a material is subjected to repeated loading and unloading.
FATS
Naturally occurring esters of glycerol (a triol) and one, two, or three fatty acids. (Fatty acids are carboxylic acids with a long hydrocarbon chain). Fats are a source of energy in foods.
FERMENTATION
A group of chemical reactions induced by microorganisms or enzymes that split complex organic compounds into relatively simple substances. Microorganisms like yeast and bacteria usually play a role in the fermentation process, creating for example, beer, wine, bread and yogurt – yeasts can convert sugars into ethanol and carbon dioxide by fermentation.
FRANKINCENSE
Frankincense, burned as incense in ancient Mesopotamia and Egypt, could be the oldest fragrance used by humans. Despite this, it was only in 2016 that chemists successfully broke down the essential oil of frankincense into its constituent parts through a tricky multistep process of extraction and distillation. Using human sniff testers to determine which fractions of frankincense were responsible for its smell, they identified two new compounds responsible for the “old church” scent of frankincense, called olibanic acids. Synthetic versions of the molecules have been made in the lab, so they could be used by the perfume industry in the future!
FREE RADICAL (RADICAL)
Free radicals are species that have one or more unpaired valence electrons and are typically very reactive. An example is the hydroxyl radical, HO•.
FRESHLY BAKED BREAD
The organic compounds that produce the aroma of freshly baked bread are affected by the ingredients and by-products formed during fermentation, and baking, where Maillard reactions produce compounds with characteristic aromas. So, a mixture of compounds gives rise to the smell, including 2-acetyl-1-pyrroline (or 2AP), which has been described as having a smell like hot buttered popcorn. Based on the ingredients, it is perhaps not surprising that some compounds, including 3-methylbutanal (or isovaleraldehyde, (H3C)2CHCH2CHO), are found in both bread and beer.
FUNCTIONAL GROUP
An atom, or group of atoms, that has similar chemical properties whenever it occurs in different compounds. An example of a functional group is the hydroxyl group (–OH), which is present in a family of compounds called alcohols (ROH).
FURAN
A cyclic aromatic organic compound, with a five-membered ring consisting of four carbons and an oxygen, with the formula C4H4O.
G
GLUCOSE
A sugar, made during photosynthesis (from water and carbon dioxide), with the molecular formula C6H12O6, HOCH2CH(OH)CH(OH)CH(OH)CH(OH)CHO. The D-isomer of glucose, called dextrose, is found in Nature. The linear (open-chain) form of glucose cyclises to form a more stable 6-membered ring.
GRAM-NEGATIVE BACTERIA
A type of bacteria with a cell wall consisting of an outer membrane; in which are a variety of lipopolysaccharides, which surrounds a thinner peptidoglycan later. They do not stain in a gram test but can retain a safranin (red) counterstain.
GRAM-POSITIVE BACTERIA
A type of bacteria with a cell wall consisting of thick, peptidoglycan layers. They stain a violet colour in a gram test.
GRAM TEST
A chemical test used in order to distinguish between gram-positive and gram-negative bacteria by staining positive bacteria violet and using a safranin counter-stain to stain negative bacteria red.
H
HASHED LINES
Hashed lines refer to bonds to atoms that point away from you. Sometimes you will see these bonds gradually decrease in width (but they mean the same thing!). (Do not confuse them with hydrogen bonds, which are also commonly shown as hashed lines.)
HETEROLYSIS
Heterolysis the cleavage of a bond in which both electrons move onto one of the atoms, resulting in the formation of a negatively-charged anion and a positively-charged cation.
HOMOLYSIS
Homolysis is the symmetrical cleavage of a bond, in which one electron goes onto each of the atoms; hence forming two free radicals. Also known as homolytic cleavage. Homolysis of a peroxide, ROOR, forms two alkoxyl radicals, RO•.
HYDROCARBON
An organic compound containing entirely carbon and hydrogen, such as alkanes, including ethane (H3C–CH3), and alkenes, including ethene (H2C=CH2).
HYDROGEN BOND
A weak chemical bond in which a hydrogen atom of one molecule is attracted to an electronegative atom (that has a high affinity for electrons), especially a nitrogen, oxygen, or fluorine atom, usually of another molecule. It is often represented by a hashed line e.g.
H–O–H IIIIIIIII Cl– IIIIIIIII H–O–H.
HYDROLYSIS
A chemical reaction in which water is used as a reactant in order to break a functional group into its constituent parts, thus separating some part of the molecule. Hydrolysis of an ester, RCO2, (in the presence of an acid or base catalyst) forms a carboxylic acid (RCO2H) and an alcohol (ROH).
HYDROPHILIC
A molecule or part of a molecule with high polarity (it is ‘water-loving’).
HYDROPHOBIC
A molecule or part of a molecule with low polarity (it is ‘water-fearing’).
I
IMINE
A functional group containing a C=N double bond e.g. R2C=NR2 (a secondary ketimine), RCH=NR2 (a secondary aldimine), R2C=NH (a primary ketimine) or RCH=NH (a primary aldimine). An imine is typically formed by a condensation reaction (H2O is formed as a byproduct) between a primary amine and an aldehyde or ketone. Imines can rearrange to form enamines e.g. R2C=CH–NR2 (the interconversion of imines and enamines is called tautomerism). Like aldehydes and ketones, imines react with nucleophiles because the carbon atom in the C=N bond is partially positive (electrophilic).
INTERMEDIATE
A species with a lifetime appreciably longer than a molecular vibration that is formed from the reactants and reacts further to give, directly or indirectly, the products of a reaction.
ISOMERISATION REACTION
An isomerisation reaction is a reaction that converts one molecule into another molecule with a different structural arrangement, but with the same molecular formula. As both molecules have the same molecular formula they are called isomers.
IUPAC NOMENCLATURE
A systematic method of naming organic compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). From the IUPAC name, an unambiguous structure of the organic compound can be drawn. In contrast, common or trivial names, do not allow the structure to be determined. For the carboxylic acid CH3CO2H, the common name is acetic acid and the IUPAC name is ethanoic acid. More complicated structures can have long IUPAC names, for example, vitamin C (or L-ascorbic acid) has the IUPAC name (5R)-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-one (the R and S define the arrangement of groups bonded to two chiral carbon atoms).
J
K
KETONE
A ketone is the name for a group of organic compounds that contain a carbonyl group (C=O) that is bonded to two hydrocarbon groups (–CnH2n+1), RCOR. The C=O bond is located in the middle of the carbon chain; an example is pentan-3-one (shown below). It is called pentan-3-one as the C=O group is on the third carbon in the chain.
KEVLAR
Developed by Stephanie Kwolek at DuPont in 1965, this aromatic polyamide polymer is strong and flexible (it is 5 times stronger than steel, on an equal weight basis), and has uses ranging from bicycle tires (helping avoid punctures) and racing sails to table tennis bats (to increase bounce and reduce weight). Kevlar helps makes high-performance skis and ski boots lighter, stiffer, and more responsive, and helps provide improved vibration damping. It is often used in combination with other materials, forming composites.
L
LATTE ART
Latte art is a method of preparing coffee created by pouring steamed milk into a shot of espresso to give a pattern or design on the surface of the latte. Alternatively, it can be formed by “drawing” in the top layer of foam. These patterns arise because milk, foam and espresso are colloids. (A colloid is a material in which two materials that normally don’t mix are forcibly mixed. Foam is a colloid of air in water. Milk is a colloid of milk fats in water, held together by the protein casein. The espresso is a colloid of coffee solids in water.) Mixing these colloids around very carefully, by drawing the foam over the espresso and milk, gives the pattern – however, the colloids are not stable and the pattern gradually disappears. If you stir your latte, the colloids will break up even quicker.
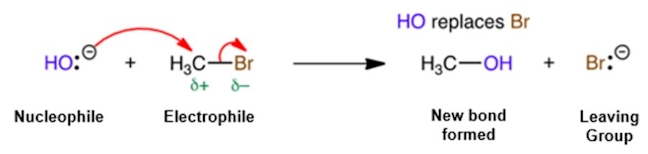
LEAVING GROUP
An atom or group (charged or neutral) that becomes detached from the main part of a reactant, which takes a pair of electrons with it. Leaving groups are anions or neutral molecules. For example, reaction of HO– with a bromoalkane (RBr) forms an alcohol (ROH), in a nucleophilic substitution reaction, where Br– acts as the leaving group. An anion that is a good leaving group must be able to stabilise the negative charge. For example, a carboxylate ion (RCO2–) is a much better leaving group than an alkoxide ion (RO–) because this anion is the more stable (a carboxylate ion is stabilised by resonance).
LIMONENE
Limonene is an essential oil found in orange peels in fairly high concentrations (the pure compound has an intense orange smell). It is a colourless liquid hydrocarbon (C10H16) used, for example, as a renewables-based solvent in cleaning products. This explains why water balloons pop when you drop the juice from an orange peel onto them (this is a good party trick!) – when the juice containing the limonene contacts the surface of the balloon, some of the rubber balloon dissolves in the limonene. (Like limonene, rubber is also a hydrocarbon and we know that ‘like dissolves like’.) This weakens the balloon and it pops. However, the choice of balloon is important – most balloons are made of vulcanised rubber, where sulfur atoms link different polymer chains, which strengthens the rubber and these do not pop when orange peel juice is squirted onto it.
LIPOPHILIC
Molecules, or parts of molecules, that dissolve in non-polar (hydrocarbon) solvents – they are ‘fat-loving’.
LOCK AND KEY HYPOTHESIS
This hypothesis broadly explains how enzyme specificity works. If an enzyme were a lock then it would have a key that was specific to that lock. Here the substrate acts as the key and so our analogy follows that only one type of substrate (key) can fit into one type of lock (enzyme). As such the binding site of the substrate must be a complimentary shape to the active site of the enzyme in order to fit correctly and form an enzyme-substrate complex, that leads to the product.
LONE PAIR
Two (paired) electrons in the outer (valence) shell of a single atom that are not part of a covalent bond. The oxygen atom in water (H2O) has two lone pairs.
LONGITUDE PRIZE
The Longitude Prize is a £10 million prize fund that will reward a competitor that can develop a point–of–care diagnostic test that will conserve antibiotics for future generations and revolutionise the delivery of global healthcare. The test must be accurate, rapid, affordable and easy to use anywhere in the world.
M
MAILLARD REACTION
Named after French chemist Louis-Camille Maillard who (at the age of just 16) studied the reaction between amino acids and sugars on heating. He suspected there was a specific reaction responsible for the change in colour of raw ingredients to dark brown, on heating – he named the brown compounds that are formed melanoidins. The products of the Maillard reaction are responsible for a food’s texture, smell, taste, shelf-life and health-value. However, despite intensive research the entire mechanisms of reactions involving amino acids, sugars and heat are not fully understood.
MARZIPAN
A sweet food containing sugar or honey and ground almonds. It is used, for example, in Christmas and wedding cakes where it is rolled into thin sheets and glazed. The aroma and flavour of marzipan can partially be attributed to benzaldehyde, PhCHO, which is found naturally in almonds. Interestingly, marzipan contains a cyanogenic glycoside (a sugar bearing a nitrile (or cyanide) group (sugar–OCH(CN)Ph), which releases benzaldehyde and poisonous hydrogen cyanide (HCN) when chewed. The body can deal with small amounts of cyanogenic glycoside, indeed, to kill a man, he would need to eat around 1 kg of marzipan in one sitting!
McCLINTOCK EFFECT
Dr Martha McClintock discovered that women living in close proximity saw their menstrual cycles synchronize – it is now known as the McClintock Effect. Also, a 2011 study found that a group of men saw their testosterone levels rise when they smelled the sweat of an ovulating woman. In another study, women were shown a sad movie and when they cried, their tears were collected. These were later held under men’s noses. Their smell didn’t elicit empathy. Instead, the men’s testosterone levels dropped. Despite these and other studies the case for human pheromones is weak. A key problem is such experiments fail to give strong evidence of a direct response to a specific chemical cue.
MECHANISM
A step-by-step description of the bond changes in a reaction, often shown using curly arrows.
MICROBIAL COLONY
Microorganisms, such as bacteria, can grow extremely fast on surfaces, when supplied with an abundance of nutrients (e.g. on the surfaces of rotten food). A colony is defined as a visible mass of microorganisms all originating from a single mother cell. Key features of these bacterial colonies serve as important criteria for their identification – some colonies may be coloured, some colonies are circular in shape, and others are irregular.
MODULUS
For elastic materials, Hooke’s Law applies, where the stress is proportional to the strain, and the modulus is defined at the ratio of stress to strain. Stress is the force causing the deformation divided by the area to which the force is applied, and strain is the measure of the deformation of the material.
MOLECULAR FORMULA
This gives the number and type of atoms present in a molecule of a substance. For example, one molecule of glucose contains 6 atoms of carbon, 12 atoms of hydrogen and 6 atoms of oxygen and so the molecular formula is C6H12O6.
MONOMER
The smallest molecular repeating unit in a polymer chain. For example, acrylic acid (H2C=CHCO2H) is the monomer used to prepare poly(acrylic acid), –[–CH2–CH(CO2H)–]n–. Poly(acrylic acid) and its derivatives are capable of absorbing many times their weight in water, which has lead to their use in disposable nappies.
MONOSACCHARIDE
Monosaccharides are the simplest carbohydrates (sometimes called single sugars) with the general molecular formula (CH2O)n, where n can be 3, 5 or 6. They are the building blocks from which all bigger carbohydrates are made.
MUSCLE FATIGUE
Muscles become fatigued (tired) during long periods of vigorous activity. This means that they stop contracting efficiently. One cause of this is the build-up of lactic acid, CH3CH(OH)CO2H, in the muscles from anaerobic respiration. The lactic acid is removed from the muscles by blood flowing through them. Interestingly, if, before exercising, sodium bicarbonate (or baking soda, NaHCO3) is taken (in small amounts) by athletes, it has been shown that it works as a buffer and neutralises small amounts of lactic acid (in an acid-base reaction). As this makes the blood less acidic (by neutralising the lactic acid) it delays fatigue and maintains the level of performance.
MUNDTICIN KS
Recently, Chinese and German researchers have found that some species of moths resist microbial infections and flourish in microbe-rich environments using bacterial species commonly found in the gut. The bacterium secretes a powerful antimicrobial peptide, called mundticin KS, killing off competitors while defending its host against pathogens. Such antimicrobial peptides could be used as food preservatives, and understanding the role of bacterial residents in the gut of insects could help develop new biocontrol strategies against herbivorous insect pests.
N
NANOTUBE
A cylindrical molecule composed of a large number of carbon atoms. Nanotubes are long and thin and shaped like tubes, about 1-3 nanometers (1 nm = 1 billionth of a metre) in diameter, and hundreds to thousands of nanometers long.
NUCLEOPHILE (LEWIS BASE)
A neutral or negatively charged species which is able to donate a pair of electrons to form a covalent bond with an atom other than hydrogen. So, it participates in a chemical reaction by donating an electron pair to form a bond to an electrophile. For example, ammonia can donate a lone pair of electrons and act as a nucleophile.
NUCLEOPHILIC SUBSTITUTION
This is a type of reaction where a nucleophile (an electron rich group, e.g. a group with a double bond or a negative charge) attacks a positive charge or a partially charged atom to form a covalent bond, kicking out a leaving group in the process.
O
OLIVE OIL
The production of olive oil involves crushing the olives and extracting the oil. The yield and flavour of olive oil depends on the variety and location of trees and on when and how the olive is picked. Green olives produce a lower yield of oil that has the most grassy and bitter flavour. Black olives give a high yield of a sweeter oil that is lower in antioxidants and shelf life but that is not very bitter. The aroma of olives and oil is chemically complex – hexanal, (E)-2-hexenal, hexan-1-ol, and 3-methylbutan-1-ol are found in most virgin olive oils. Compounds that give olive oil its fruitiness include the esters ethyl 2-methylpropanoate and ethyl 2-methylbutanoate. Aldehydes, especially (Z)-3-hexenal, give the oils a grassy note, whilst the thiol 4-methoxy-2-methyl-2-butanethiol gives a blackcurrant aroma.
ORGANIC COMPOUND
Any compound containing a carbon atom, or atoms, covalently bound to other atoms. By convention, carbonates, hydrogencarbonates, cyanates and isocyanates are classed as inorganic compounds.
OXIDATION
Oxidation is the process by which a molecule is oxidised; this occurs when a molecule loses electrons or when oxygen is added to, or hydrogen is removed from, a compound. For example, conversion of ethanol (CH3CH2OH) into ethanoic acid (CH3CO2H) is an oxidation reaction.
P
PENICILLIN
A large class of antibiotic derived directly or indirectly from strains of fungi. Their structure contains a β-lactam ring, which plays a crucial role in inhibiting bacterial cell wall synthesis. They are used to treat many types of infections, including pneumonia, gonorrhoea, and infections caused by streptococci and staphylococci.
PENICILLIN BINDING PROTEINS (PBPs)
The enzymes through which peptidoglycan cross linking occurs. Penicillin binds to the active sites of these enzymes and prevents them from cross linking individual peptidoglycan chains together to form bacterial cell walls. Without strong cell walls these bacteria are vulnerable and will die.
PEPTIDOGLYCAN
The interlinking, natural polymer that makes up the cell walls of bacteria. The individual polymer chains cross-link by joining their amino acid chains together, making them stronger and more durable. Many antibiotics act by disrupting or inhibiting this cross-linking. As such the cell wall is weakened and ruptures, killing the bacterial cell.
PHYTOHORMONE
Chemicals that regulate plant cell processes. They are signal molecules produced within the plant functioning in extremely low concentrations. For example, ethene (H2C=CH2) is produced by many different fruit varieties, such as tomatoes, apples, pears and bananas. Most fruits produce increased amounts of ethene as they ripen. For example, placing a banana in a paper bag traps the ethene around the banana and speeds the ripening process. You may remember that several species of tomatoes (e.g. Flavr Savr) were genetically engineered in the 1990s to ripen more slowly – the altered gene sequence decreased the production of ethene (avoiding spoilage during storage and transportation for longer periods than normal tomatoes). They soon disappeared from other shelves due to supply problems, mixed taste-reviews and marketing controversies.
POLYAMIDE
A macromolecule with repeating units linked by amide bonds, –NHCO– or –NRCO–. Natural polyamides include proteins, while synthetic polyamides include Kevlar and nylon. Nylon polymers are formed in industry using condensation reactions between equal amounts of diamines and dicarboxylic acids or diacyl chlorides. Wallace Carothers made the first example of a nylon, called nylon 6,6, which is a copolymer of a 6-carbon diamine and a 6-carbon dicarboxylic acid or diacyl chloride. Starting materials with different carbon chain lengths can be used to form nylons with different physical properties (e.g. toughness). When a solution of decanedioyl dichloride in cyclohexane floats on an aqueous solution of 1,6-diaminohexane, nylon 6,10 forms at the interface. It can be pulled out as fast as it is produced forming a long thread – in the ‘nylon rope trick’.
POLYMER
A long molecule consisting of many, smaller, molecular repeating units known as monomers. Polymers have a wide range of applications in areas such as construction, medicine, sports, performance engineering and foodstuffs.
POLYPEPTIDE
A ‘polypeptide chain’ makes up part, or the entirety, of a protein molecule and consists of several amino-acid residues that are bonded together to form a chain.
POLYPHENOL
Organic compounds containing two or more benzene rings that each have at least one hydroxyl group (OH) attached to the ring(s). Many polyphenols occur naturally in plants and some kinds, such as the flavonoids and tannins, are believed to be beneficial to health (chiefly as antioxidants).
POLYSACCHARIDE
Polymeric carbohydrates made up of long chains of monosaccharides joined together by acetal groups (called glycosidic linkages). Examples include cellulose and starch. Starch is a polymer of glucose units, which is produced in most green plants as an energy store.
PPB
Stands for parts per billion. It denotes one part per 1,000,000,000 parts, or one part in 109. 1 ppb corresponds to 1 μg/L.
PRONTOSIL
An azo dye (orange-red in colour), containing a sulfonamide functional group, that has antimicrobial properties.
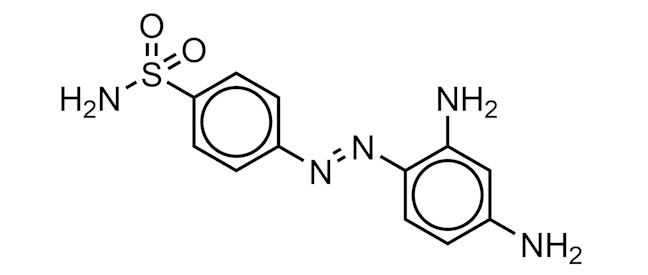
It was the first of a class of antibiotic medicines called sulfa drugs. When Prontosil enters the body it is broken down into smaller pieces. One of these pieces, called sulfanilamide (4-aminobenzenesulfonamide), is the active medicine responsible for killing bacteria.
PROPHYLAXIS
The action of taking steps to prevent a possible infection or disease before it occurs.
PROTEIN
A class of organic compounds, which are large molecules composed of one or more long chains of amino acids and are an essential part of all living organisms (a linear chain of amino acid residues is called a polypeptide). Many proteins are enzymes that catalyse biochemical reactions and are vital to metabolism (i.e. the life-sustaining chemical reactions within the cells of living organisms).
PYRROLE
A cyclic aromatic organic compound, with a five-membered ring consisting of four carbons and a nitrogen atom, with the formula C4H4NH.
Q
R
R AND S CONFIGURATION
This nomenclature (sometimes called the CIP nomenclature) is used to indicate the 3-dimensional arrangement (or configuration) of all atoms or groups attached to a chiral carbon atom. Each chiral center in a molecule is assigned a prefix (R or S), according to whether its configuration is right- or left-handed. The assignment uses a system that ranks the atoms or groups attached to the chiral centre.
RACEMATE
A mixture of equal amounts of enantiomers.
RADICAL INITIATION
A radical initiation reaction is the process of forming free radicals from an uncharged species. This occurs when a covalent bond undergoes homolytic cleavage (the symmetrical cleavage of a bond, where one electron goes onto each of the atoms between which the bond is broken), thus forming two free radicals.
RADICAL PROPAGATION
Propagation reactions are radical reactions where radicals react to form a different radical.
RADICAL TERMINATION
A radical termination reaction occurs when radicals are destroyed and are turned into less reactive (non-radical) species; i.e. when two radicals react together to form a covalent bond.
RECEPTOR
A molecular structure or site on the surface or interior of a cell that binds with substances such as hormones, medicines, or neurotransmitters.
REDUCTION
Reduction is the process by which a molecule is reduced; this occurs when a molecule gains electrons or when hydrogen is added to, or oxygen is removed from, a compound. The conversion of ethanoic acid (CH3CO2H) into ethanol (CH3CH2OH) is a reduction reaction.
RESONANCE
Resonance occurs when a molecule with delocalised electrons is more accurately described by two or more structures. The extra stability gained by the delocalisation of electrons is called the resonance energy.
S
SAFRANIN
A biological stain used to counterstain gram-negative bacteria in the gram test.
SALVARSAN
The original compound discovered by Paul Elrich to be effective in treating syphilis infections.
SATURATED
A compound with no double or triple bond. For example, ethane (H3C–CH3) is a saturated compound.
SKELETAL STRUCTURE
The full structural formula of large molecules can get really cluttered; therefore, to make the molecule easier to see, many of the atoms can be removed. For example, in pentan-3-one (shown below), only the symbol for the oxygen atom is highlighted. Each end of a straight line represents a carbon atom, unless another atom is shown, and many, if not all, of the hydrogen atoms are not shown.
STARCH
Starch is a polymeric carbohydrate consisting of a large number of glucose units joined together. This polysaccharide is produced by most green plants as an energy store.
STEAM DISTILLATION
A separation technique often used for temperature sensitive organic compounds. Steam is introduced into the distillation apparatus and the water vapour carries small amounts of low-boiling organic compounds into a condenser, where the condensed liquid phase separates, allowing for easy collection.
STEREOCHEMISTRY
The study of the spatial relationship of atoms within a molecule. Stereoisomers are isomers that differ in the way their atoms are arranged in space (enantiomers and diastereomers are stereoisomers).
STERIC EFFECT
The effect on a molecule or reaction due to the size of atoms. Steric hindrance occurs when bulky groups at the site of a reaction make it difficult for the reactants to approach each other.
STRAIN
A molecule experiences strain when there is a distortion of bond lengths and bond angles from their ideal values. The internal energy of a molecule consists of all the energy stored within it. A strained molecule has an additional amount of internal energy, which an unstrained molecule does not.
STRUCTURAL ISOMERS
Molecules that have the same molecular formula but differ in the way the atoms are connected. Structural isomers can be divided into chain isomers, position isomers and functional group isomers.
Butane and 2-methylpropane are chain isomers – they both have the molecular formula C4H10, but as the carbon chains are connected in different ways they are called chain isomers.
1-Bromopropane and 2-bromopropane are position isomers – they both have the molecular formula C3H7Br, but 1-bromopropane has the bromine atom at the end of the chain, whereas 2-bromopropane has the bromine atom in the middle of the chain.
Propanal and propanone both have the molecular formula C3H6O, but as one is an aldehyde and the other a ketone, they are called functional group isomers.
STRUCTURE ACTIVITY RELATIONSHIP (SAR)
SAR is method through which the properties of a molecule can be examined by comparing its structure to that of similar molecules. Molecules with similar shapes may be found to target similar biochemical pathways in the body by, for example, interacting with the same enzyme. The removal or modification of key functional groups in a molecule can also be undertaken in order to identify which areas of the molecule are responsible for its active properties.
SUBSTITUENT
An atom or group, other than hydrogen, in a molecule.
SUBSTITUTION REACTION
A reaction in which an atom or group is replaced by another atom or group. For example, hydrolysis of a bromoalkane, RBr, using HO–/H2O, forms an alcohol (ROH) in a nucleophilic substitution reaction.
SUBSTRATE
The molecule that is acted upon by an enzyme. It is often altered in some way before being released from the enzyme. The substrate must have a binding site which is a complimentary shape to that of the enzyme active site in order for the two to interact in an enzyme-substrate complex.
SUGAR
Sweet short-chain, soluble carbohydrates, commonly used in food and drink. There are different sugars from a range of sources, for example, table (or granulated) sugar is the disaccharide sucrose (made by combining the monosaccharides glucose and fructose), which is extracted and refined from cane and beet sugar. It has the formula C12H22O11 – to emphasise it is a hydrate of carbon, it can be written as C12(H2O)11. If the water is removed from sugar, all that is left behind is carbon and this can be demonstrated using concentrated sulfuric acid. Sulfuric acid is a dehydrating agent that removes water from the sugar, leaving behind black elemental carbon in a dramatic fashion (forming a carbon ‘snake’).
T
TANNINS
Substances with an astringent taste, found widely in plants, and used in, for example, tanning leather. They typically have a complex structure containing a number of phenol groups (i.e. they are polyphenols). The astringency from the tannins is what causes the dry and puckery feeling in the mouth following the consumption of unripened fruit, red wine or tea.
TAUTOMERISM
The ability of certain organic compounds to react in isomeric structures that differ from each other in the position of a hydrogen atom and a double bond e.g. CH3C(=O)CH3 and CH3C(OH)=CH2 is called keto-enol tautomerism; the compound containing the C=O bond is called the keto form, and the one containing C(OH)=C is called the enol form.
TENSILE STRENGTH
The capacity of a material or structure to withstand loads tending to elongate or stretch it, as opposed to compressive strength, which withstands loads tending to reduce size. Tensile strength is an important measure of a material’s ability to perform in an application.
TETRAHEDRAL
The angles of the four groups around the central carbon are approximately 109° to each other and result in a tetrahedral arrangement around the central carbon.
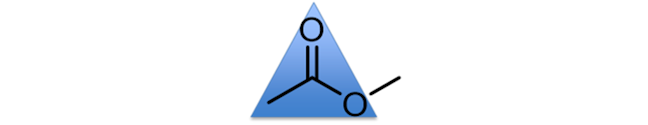
TRIGONAL PLANAR
An example of a trigonal planar unit is the C-(C=O)-O unit (of an ester), which has bond angles of approximately 120°. You could consider the central carbon atom as the central point in a triangle, with the other three atoms pointing to the corners of a triangle.
U
UNSATURATED
Unsaturated compounds are compounds that contain one or more double or triple bonds. For example, alkenes (e.g. RCH=CHR) and benzene are unsaturated compounds.
V
VULCANISATION
Vulcanisation is the process by which rubber molecules (polymers or macromolecules made of repeating units or monomers called isoprene) are cross-linked with each other by heating the liquid rubber with sulfur. Cross-linking increases the elasticity and the strength of rubber by about ten-fold, but the amount of cross-linking must be controlled to avoid creating a brittle and inelastic substance. The process of vulcanisation was discovered accidentally in 1839 by the American inventor Charles Goodyear (1800-1860) when he dropped some rubber containing sulfur onto a hot stove.
W
WEDGED LINES
Wedged lines refer to bonds to atoms that point towards you. Whereas straight thin lines refer to bonds to atoms that are in the plane of the page.
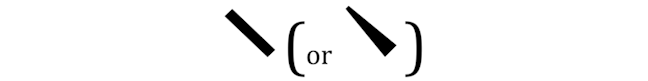
X
X-RAY CRYSTALLOGRAPHY
The method through which the structures of molecules can be determined by measuring the angles and intensities at which x-ray light, which is shone onto the crystalline molecule, is diffracted.
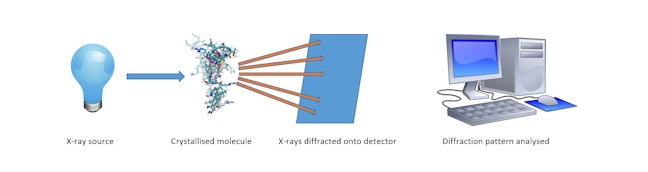
X-ray crystallography of biological molecules originated with Dorothy Crowfoot Hodgkin, who solved the structures of cholesterol (1937), penicillin (1945) and vitamin B12 (1956), for which she was awarded the Nobel Prize in Chemistry in 1964.
Y
Z
Share this

Reach your personal and professional goals
Unlock access to hundreds of expert online courses and degrees from top universities and educators to gain accredited qualifications and professional CV-building certificates.
Join over 18 million learners to launch, switch or build upon your career, all at your own pace, across a wide range of topic areas.
Register to receive updates
-
Create an account to receive our newsletter, course recommendations and promotions.
Register for free








