How do antibiotics work?
In the previous Steps you’ve learned how pathogens are transmitted, the symptoms they cause and how to diagnose an infectious disease. Having identified the culprit of an infection, the next step is to treat the patient with an appropriate antimicrobial agent (if one is available).
Antimicrobials are chemicals that either kill microbes or prevent their growth. Disinfectants (eg bleach), and antiseptics (eg iodine, chlorhexidine) are non-selective antimicrobials that kill a wide range of different microbes. Whilst disinfectants can be used to decontaminate surfaces to prevent the spread of infectious diseases and antiseptics help prevent infections during surgery, neither can be used internally to treat infectious diseases as they are also toxic to humans. Selective antimicrobials target particular functions in particular groups of microbes: antibiotics target certain types of bacteria; antifungals target certain fungi; antiparasitics target specific types of protists (eg antimalarials target Plasmodium, the protist that causes malaria); and antivirals inhibit the replication of specific viruses. Their specificity for microbes means they can be safely used to treat infectious diseases without serious side effects in humans.
With all these different types of antimicrobials, it is not surprising that there are many different mechanisms of antimicrobial action and microbial resistance. In this Step we will focus on antibiotics.
Antibiotics: mode of action
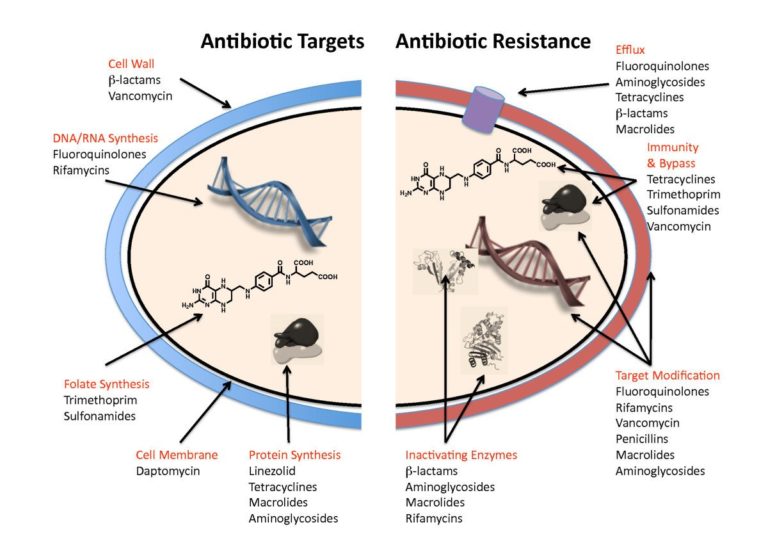
Antibiotics act by disrupting a specific cellular component (eg cell wall, cell membrane) or biosynthetic pathway (protein synthesis, nucleic acid synthesis, folate synthesis) within a bacterial cell (Figure 1). Penicillin is a Beta-lactam antibiotic which works by inhibiting an enzyme that cross-links peptidoglycan layers in the bacterial cell wall. It is specific to bacteria because only bacteria have this polymer in their cell wall, and it is more effective against Gram positive bacteria because they have a much thicker layer of peptidoglycan in their cell wall than Gram negative bacteria. When a bacterial cell replicates, it makes small cuts in the peptidoglycan layer in order to divide and to incorporate more peptidoglycan into the cell wall as it grows. Penicillin inhibits the enzyme, called penicillin binding protein (PBP), that forms the cross-links in the peptidoglycan layer. When a bacterial cell replicates in the presence of penicillin the peptidoglycan layer becomes weaker and weaker, and the cell ultimately bursts when it can no longer withstand the internal osmotic pressure.
Figure 1: Diagram to summarise the main targets of antibiotics in bacterial cells, and the main mechanism of antibiotic resistance © Gerard D Wright via Wikimedia 2.0 Generic (CC BY 2.0)
Antibiotic resistance mechanisms
Bacteria use a number of different mechanisms to defend themselves against the toxic effects of antibiotics (Figure 1). They may produce a slightly modified cellular component that is no longer inactivated by the antibiotic (target modification), or they may produce an inactivating enzyme that modifies or destroys the antibiotic or produce an entirely new enzyme that is not sensitive to the antibiotic to bypass the inactivated component. Alternatively, resistance can be achieved by reducing the ability of the antibiotic to reach the target by either decreasing uptake of the antibiotic or actively removing it from the cell using an efflux pump.
Penicillin resistance in Gram positive bacteria is predominantly via target modifications that prevent penicillin from binding to the PBP, whereas resistance in Gram negative bacteria is more commonly achieved by production of inactivating enzymes called (beta)-lactamases.
The genetic basis of resistance
In Step 1.11, you read how bacteria replicate asexually by binary fission, where one bacterial cell splits in half to produce two almost identical daughter cells (this is also referred to as vertical gene transfer, because genes are passed down the generations from parent to offspring). The reason there is an “almost” in the previous sentence is because one of the daughter cells will probably have picked up one or a few mutations in its genome as a result of mistakes during DNA replication. Such mutations may happen to confer resistance to an antibiotic if, for example, it alters the structure of the antibiotic target (target modification) or reduces uptake of the antibiotic by changing the membrane permeability (eg altering a porin).
So how do bacteria produce a totally new enzyme to inactivate or bypass the antibiotic, or make a new efflux pump? It may take millions of generations for a new enzyme to evolve via mutation and natural selection, but bacteria have a way to speed things up…they are able to exchange genes with each other via a process called horizontal gene transfer (HGT). During HGT copies of one, a few or many genes are transferred from one fully formed bacterial cell to another fully formed bacterial cell (hence the term horizontal). If the new genes help bacteria to survive and replicate in adverse conditions (eg presence of antibiotics) these genes will spread rapidly between different species within a microbial community (Figure 2).
Figure 2: Examples of how antibiotic resistance spreads © Centers for Disease Control and Prevention [public domain]
The more antibiotics are used, the greater the benefit resistance genes provide to the bacteria and the more the resistance genes spread by HGT within the community. The WHO have declared that antimicrobial resistance (AMR) is one of the biggest health crises we face today, and they predict antimicrobial resistant infections will be the leading cause of death worldwide by 2050.
You can find out more about how HGT works in this additional resource pdf. You may also like to revisit the Microbes at war animation you watched in Week 1.
Why is it important to complete a full course of antibiotics if prescribed by your health professional? Why do you think there has been a decrease in the number of new antibiotics under development in recent years, despite the alarming increase in AMR infections? Share your thoughts in the comment area below.
Recommended reading
-
Q&A: Antibiotic resistance: where does it come from and what can we do about it? Wright; licensee BioMed Central Ltd. 2010)
-
Livermore, D.M. Globalisation of antibiotic resistance. Microbiology today. Nov 8th, 2016.
Share this
Small and Mighty: Introduction to Microbiology
Small and Mighty: Introduction to Microbiology


Reach your personal and professional goals
Unlock access to hundreds of expert online courses and degrees from top universities and educators to gain accredited qualifications and professional CV-building certificates.
Join over 18 million learners to launch, switch or build upon your career, all at your own pace, across a wide range of topic areas.
Register to receive updates
-
Create an account to receive our newsletter, course recommendations and promotions.
Register for free

 Click to expand
Click to expand





