Chemical hazards that affect occupational health

Share this step
Lead
Exposure
Exposure to inorganic lead can occur in metal foundries and smelters, in battery factories, when removing lead paint, when welding and cutting metal parts coated with lead paint, when producing items made of enamel, brass, tin and bronze, recycling of lead-containing waste, in manufacturing of glass, ceramics and certain types of plastics and when producing ammunition. Uptake of inorganic lead in the body is mainly through inhalation and ingestion. Examples of organic lead compounds are tetraethyl lead and tetramethyl lead, which are used to make leaded gasoline. Organic lead compounds are absorbed as a vapour by inhalation and are also readily absorbed through the skin.
Metallic lead and lead compounds, both inorganic and organic, are toxic, and some of them are carcinogenic. Metal oxide formed on the surface of metallic lead is powder-like, and when touched by the workers can be easily transferred to the fingers, and might thereby be ingested when eating or smoking. Thus, personal hygiene, and especially hand washing, is very important when handling lead.
Reductions and prohibitions in the use of lead in petrol, paint, plumbing and solder in many countries have resulted in substantial reductions in blood lead levels. However, significant sources of exposure still remain, particularly in developing countries.
The Occupational Exposure Limits for lead in the workplace atmosphere is low. For instance, the TLV set by the ACGIH is 0.05 mg/m3.
The Biological Exposure Index (BEI) of ACGIH for lead in blood is 30 μg/100 ml. Due to the long half-life of lead in blood, the sampling timing is not critical. ACGIH has a note concerning women with child bearing potential stating that when the lead blood of the woman exceeds 10 μg/100 ml, there is a risk of delivering a child with blood lead over the current US Centers for Disease Control guidance of 10 μg/100 ml. The binding biological limit value of the European Union is 70 μg Pb/100 ml blood
 Bags with a powder mix which contains lead.
Bags with a powder mix which contains lead.
© G. Tjalvin
Health effects
Inorganic lead is a cumulative toxicant that affects multiple body systems, including the neurologic, hematologic, gastrointestinal, cardiovascular, and renal systems. If lead stores in the body are not high due to previous lead exposure the biological half-life of lead in blood is normally about one month. Lead is stored in the skeleton, and the half-life of lead in the skeleton is long, 10 years or more.
For inorganic lead, the following health effects may occur:
Mercury
Exposure
Mining
 Mercury in liquid form is used in the amalgamation process in gold mines. © Paul Israel Nyarubeli
Mercury in liquid form is used in the amalgamation process in gold mines. © Paul Israel NyarubeliDental offices
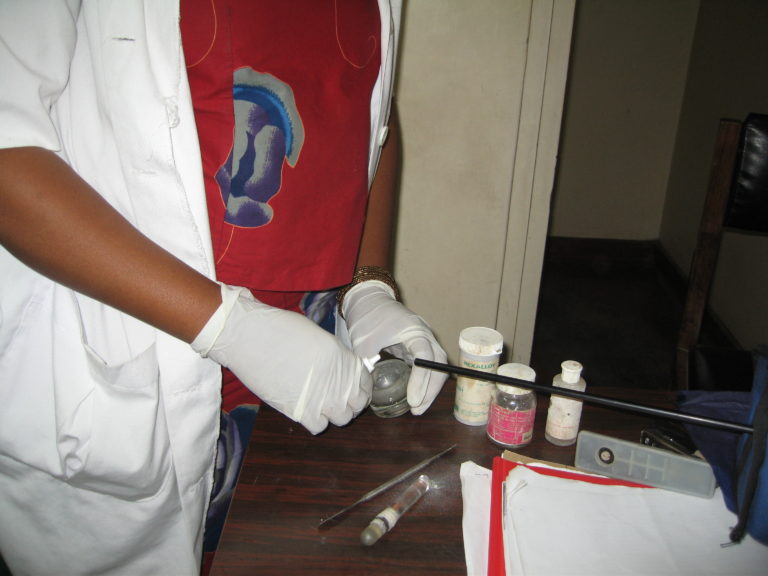 Dental assistant preparing amalgam containing mercury. Mercury in the air is monitored by a direct-reading instrument. © B.E. Moen
Dental assistant preparing amalgam containing mercury. Mercury in the air is monitored by a direct-reading instrument. © B.E. Moen Heating of mercury for amalgamation may cause high exposures in dental offices. © N.R. Gjerdet
Heating of mercury for amalgamation may cause high exposures in dental offices. © N.R. GjerdetHealth effects
Inorganic mercury
Organic mercury – Minemata disaster
 A rehabilitation centre for patients poisoned at Minemata. Several of the patients cannot walk, but are dependent on a wheel chair. © Akwilina V. Kayumba
A rehabilitation centre for patients poisoned at Minemata. Several of the patients cannot walk, but are dependent on a wheel chair. © Akwilina V. Kayumba
An example of organic mercury exposure affecting public health occurred in Minamata, Japan, between 1932 and 1968, where a factory producing acetic acid discharged waste liquid into Minamata Bay. The discharge included high concentrations of methylmercury. The bay was rich in fish and shellfish, and provided the main livelihood for local residents and fishermen. At least 50 000 people were affected to some extent and more than 2000 cases of Minamata disease were certified. The most severe cases suffered brain damage, paralysis, incoherent speech and delirium.
Acute exposure to very high levels of methyl mercury results in CNS effects such as blindness, deafness, and impaired levels of consciousness.
Cadmium
Exposure
Workers may be exposed to cadmium in the zinc, copper and steel industries, in the manufacture of nickel-cadmium batteries, solar cells, and jewelry, in metal plating, welding of cadmium-containing metals, production of plastics and many other industrial activities. The Occupational Exposure Limits for cadmium in the workplace atmosphere is low. The TLV set by the ACGIH for the cadmium is 0.01 mg/m3.
Health effects
The main route of cadmium exposure in the occupational setting is via the respiratory tract. Cadmium is toxic when inhaled, and is classified as carcinogenic to humans by the International Agency on Cancer (IARC). Cadmium accumulates in the kidney cortex, and may cause progressive renal disease. A characteristic low molecular weight protein marker is found as a result of kidney damage by cadmium. Cadmium exposure may also have effects on calcium-metabolism and thereby reduce the bone-density resulting in an increased risk of fractures.
The Biological Exposure Index (BEI) of ACGIH for cadmium in urine is 5μg/g creatinine. Due to the long half-life of cadmium, the urine sampling timing is not critical. Cigarette smoking is a significant source of cadmium, and needs to be taken into account when interpreting the results .
Share this
Occupational Health in Developing Countries

Occupational Health in Developing Countries


Reach your personal and professional goals
Unlock access to hundreds of expert online courses and degrees from top universities and educators to gain accredited qualifications and professional CV-building certificates.
Join over 18 million learners to launch, switch or build upon your career, all at your own pace, across a wide range of topic areas.
Register to receive updates
-
Create an account to receive our newsletter, course recommendations and promotions.
Register for free







