Challenges of AMS in the Haematology Oncology Department
Share this step
Dr Mushira Enani will discuss the challenges and solutions associated with AMS in the haematology oncology department, and immunocompromised patients.
Patients with acute myeloid leukaemia (AML) and those undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT) are at highest risk of developing profound and prolonged (lasting >10 days) neutropenia. As a result, this group is vulnerable to bacterial, fungal, viral and parasitic infections, the only sign of which may be a fever.
Management of febrile neutropenia includes early initiation of empirical and usually multiple extended courses of broad-spectrum antimicrobials to prevent early mortality. However, this approach may predispose haematology oncology patients to adverse events, infection with multidrug-resistant organisms (MDROs), and C. difficile infection.
This provides an exclusive opportunity to implement AMS in this population: aiming to optimise antimicrobial use by ensuring proper selection, dose, route, and duration; and improving patient’s outcome and reducing infection and colonisation by MDROs and development of C. difficile infection.
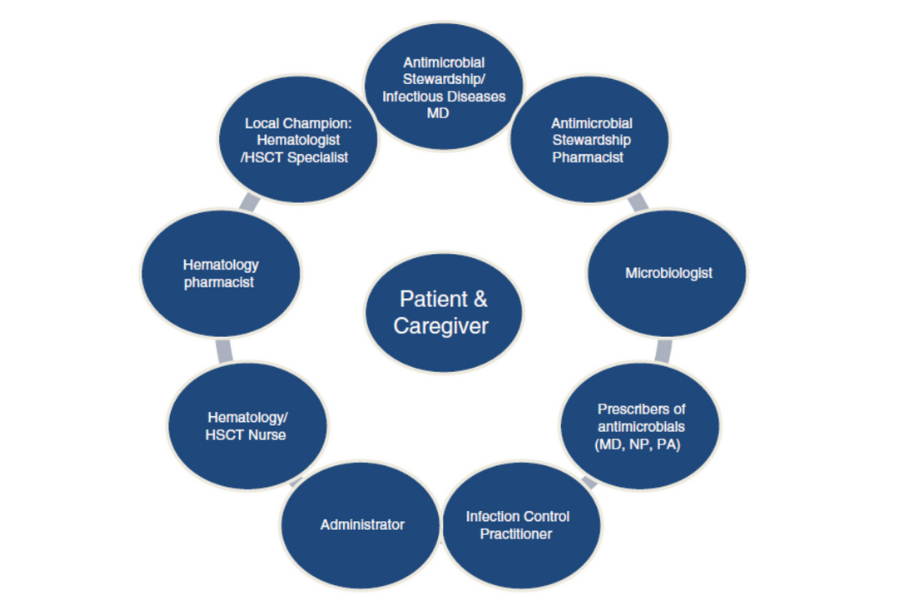
Many studies have proven the safety and cost-effectiveness of ASPs in haematology oncology patients. Current evidence supports the feasibility, effectiveness and safety of antimicrobial stewardship programs for malignant haematology and hematopoietic stem cell transplant patients. Such programs for immunocompromised populations are most successful when a multidisciplinary team approach is used.
Role of the AMS team in immunocompromised hosts
- Produce and distribute updated antimicrobial susceptibility trends for common organisms (antibiogram) to health care providers
- Formulary review
- Pre-authorisation of restricted antimicrobial before prescription (front-end strategy)
- Prospective audit with intervention and feedback after prescription (back-end strategy)
- Monitoring drug interactions
- Renal dosing optimisation
- Streamlining and discontinuation of unnecessary antimicrobials
- Antifungal stewardship
- Antiviral stewardship
- Develop multidisciplinary protocols, algorithms, and guidelines for the diagnosis, management, and prophylaxis of common infections
- Discuss microbiology, antibiotic use, and infection-related outcome data in multidisciplinary committees
In a retrospective Italian study by Gustinetti et al, de-escalation and discontinuation of antimicrobial therapy was feasible in the pre-engraftment phase of allogenic HSCT with similar fever relapses and mortality rates compared with the non-de-escalation group.
“Developments in Emerging and Existing Infectious Diseases” has a chapter titled Antimicrobial Stewardship in Haematology Patients, which highlights the 10 Basic Principles of Antimicrobial Stewardship in Haematology Patients With Febrile Neutropenia as follows:
- Local data availability: current epidemiology of bacteremia and other infections and the outcome data (e.g., infection-related mortality and length of hospital stay) should be readily available.
- Early diagnosis: Early microbiological diagnostic procedures should be implemented, and the results should immediately be communicated to the clinicians.
- Prophylaxis: Oral flouroquinolone prophylaxis can be used in selective high-risk neutropenic patients (see text for eligibility). Surveillance for the emergence of resistance should be maintained during prophylaxis. The prophylaxis should be discontinued once the patient recovers from neutropenia or becomes febrile and the empirical therapy is initiated.
- Risk stratification: The risk stratification and local epidemiology should prompt the type of empirical therapy (e.g. oral vs parenteral, inpatient vs outpatient, and escalation vs de-escalation).
- Colonisation of resistant bacteria: Previous colonisation with MDR bacteria should guide the choice of initial empirical therapy.
- De-escalation and switch therapy: Once microbiology data become available, de-escalation to a narrower spectrum of antibiotics or switch to oral therapy should be provided.
- Duration of therapy: Empirical or targeted therapy should not be prolonged until recovery from neutropenia and may be discontinued earlier in stable patients (see step 2.14 for further details on DOT).
- Infection control: Enteric decolonisation is discouraged in MDR-colonised neutropenic patients. But basic infection control principles must meticulously be applied.
- Team-based approach: A team-based approach should be provided when managing patients with febrile neutropenia. The team members should include but not restricted to a haemato-oncologist, infectious disease physician, clinical microbiologist and a pharmacist, if available.
- Guideline adherence: Management protocols prepared according to the local epidemiologic data should be provided and guide the physicians in charge of these patients.
An example of management strategies for low- and high-risk febrile neutropenic patients can be found here.
Challenges to the implementation of antimicrobial stewardship in immunocompromised hosts
Although the need and impact of ASP is clear, some challenges exist that may hamper AMS team efforts in haematology oncology patients, such as:
- Physician perceptions and attitudes “my patient is sicker than yours”
- Wide range of possible infectious etiologies with diagnostic uncertainty
- Impaired inflammatory responses
- Difficulty in making an early diagnosis
- Urgency for empiric effective antimicrobial therapy
- Significant drug toxicities and potent drug interactions
- Prolonged exposure to prophylactic antibiotics may lead to antimicrobial resistance
- Increasing antimicrobial resistance with limited therapeutic options to appropriately treat empirically or documented infections
- Difficulty with distinguishing rejection and graft versus host disease from infections
- Difficulty in controlling the source of infection due to issues, such as thrombocytopenia, limiting surgical interventions
- Prolonged duration of immunosuppressed state increases the risk for uncommon presentations of common and uncommon infections
- Duration of antimicrobial therapy not clearly defined in many infections for these patients
Understanding prescribers’ attitudes, perceptions, and behaviours is particularly important when providing recommendations in immunocompromised patients. The AMS team members need to be visible in the patient’s clinical areas, fully dedicated for stewardship activities, have superb communication skills, and consistent in their approach so as to build collaboration and trust with prescribers.
Other tactics to overcome barriers include utilisation of rapid infection diagnostics – this overcomes diagnostic uncertainty and enables appropriate management of specific organisms in a timely manner, which limits unnecessary antimicrobial use.
Robust leadership support (authorisation and advocacy) and sustained support from local clinical leaders in haematology-oncology to champion the AMS is essential for successful implementation and sustainability. The presence of the haematology-oncology champion facilitates collaboration/trust between infectious diseases and haematology oncology consultants to establish/update best practice guidelines and clinical pathways. Spreading awareness and education on AMS to frontline staff, nurses, pharmacists, and patients with haematology-oncology diseases will further support stewardship efforts.
Data sharing and feedback to prescribers is among the best strategies to drive change in prescribing behaviour. Data may include one or more of the following: quarterly antibiotic utilisation (DDD/DOT) and microbial resistance trends, C. difficile rate, compliance with AMS recommendations, rate of de-escalation or discontinuation, average duration of empirical antimicrobial therapy, and patient’s outcome and length of hospital stay. Measuring economic impacts (ROI) after implementing ASP is an added benefit and an outcome measure that is particularly attractive to senior leaders particularly in the face of improved patient’s outcome.
Some examples of how to display antibiotic use data and other stewardship metrics can be found in the Canadian SHS & UHN Antimicrobial Stewardship Program Q4 report.
BSAC have a FutureLearn course on Antifungal Stewardship if you wish to learn more about this topic.
Share this
Antimicrobial Stewardship for the Gulf, Middle East and North Africa

Antimicrobial Stewardship for the Gulf, Middle East and North Africa


Reach your personal and professional goals
Unlock access to hundreds of expert online courses and degrees from top universities and educators to gain accredited qualifications and professional CV-building certificates.
Join over 18 million learners to launch, switch or build upon your career, all at your own pace, across a wide range of topic areas.
Register to receive updates
-
Create an account to receive our newsletter, course recommendations and promotions.
Register for free







