Home / Healthcare & Medicine / Bioinformatics / Making sense of genomic data: COVID-19 web-based bioinformatics / Variant calling and annotation
This article is from the free online
Making sense of genomic data: COVID-19 web-based bioinformatics


Reach your personal and professional goals
Unlock access to hundreds of expert online courses and degrees from top universities and educators to gain accredited qualifications and professional CV-building certificates.
Join over 18 million learners to launch, switch or build upon your career, all at your own pace, across a wide range of topic areas.

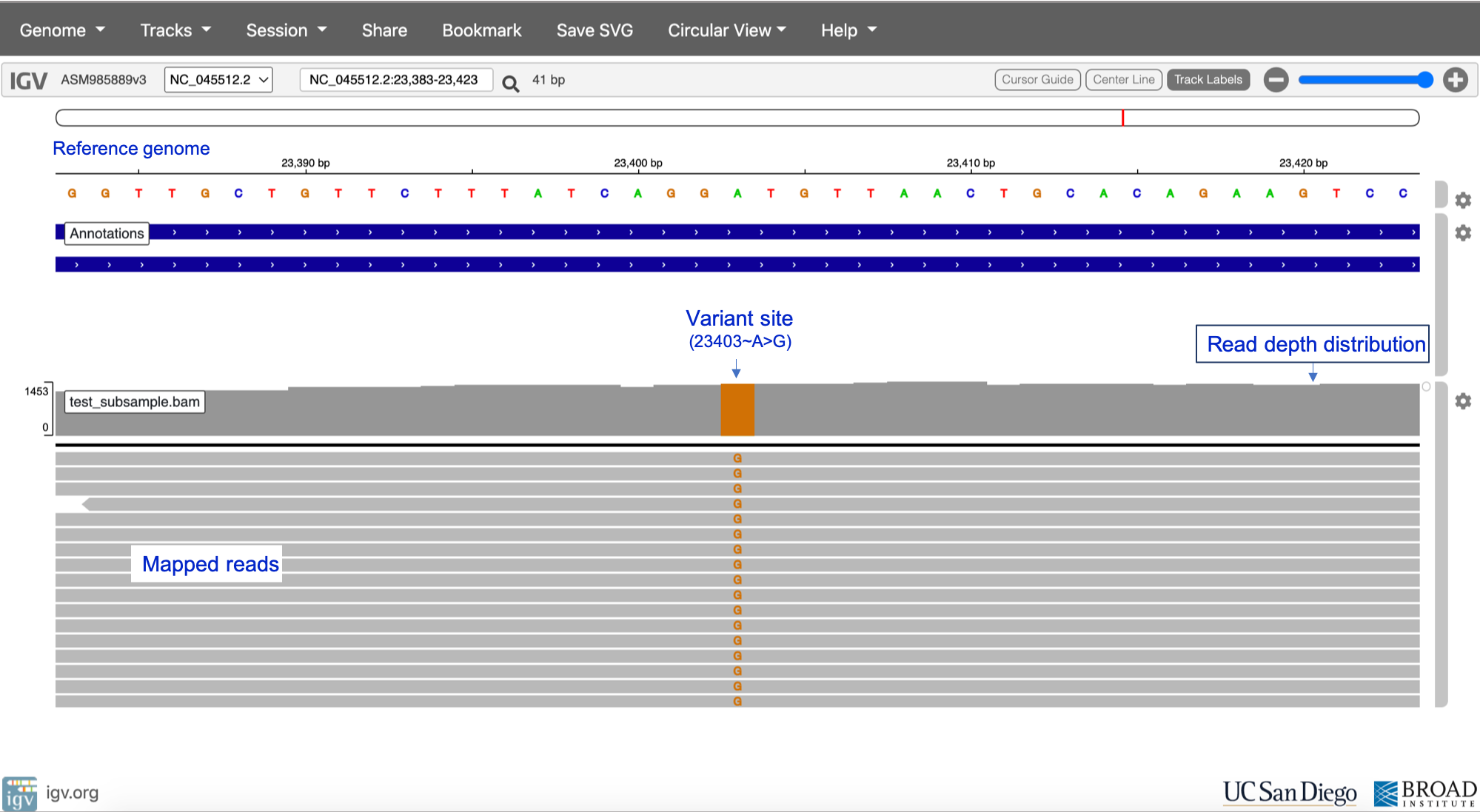
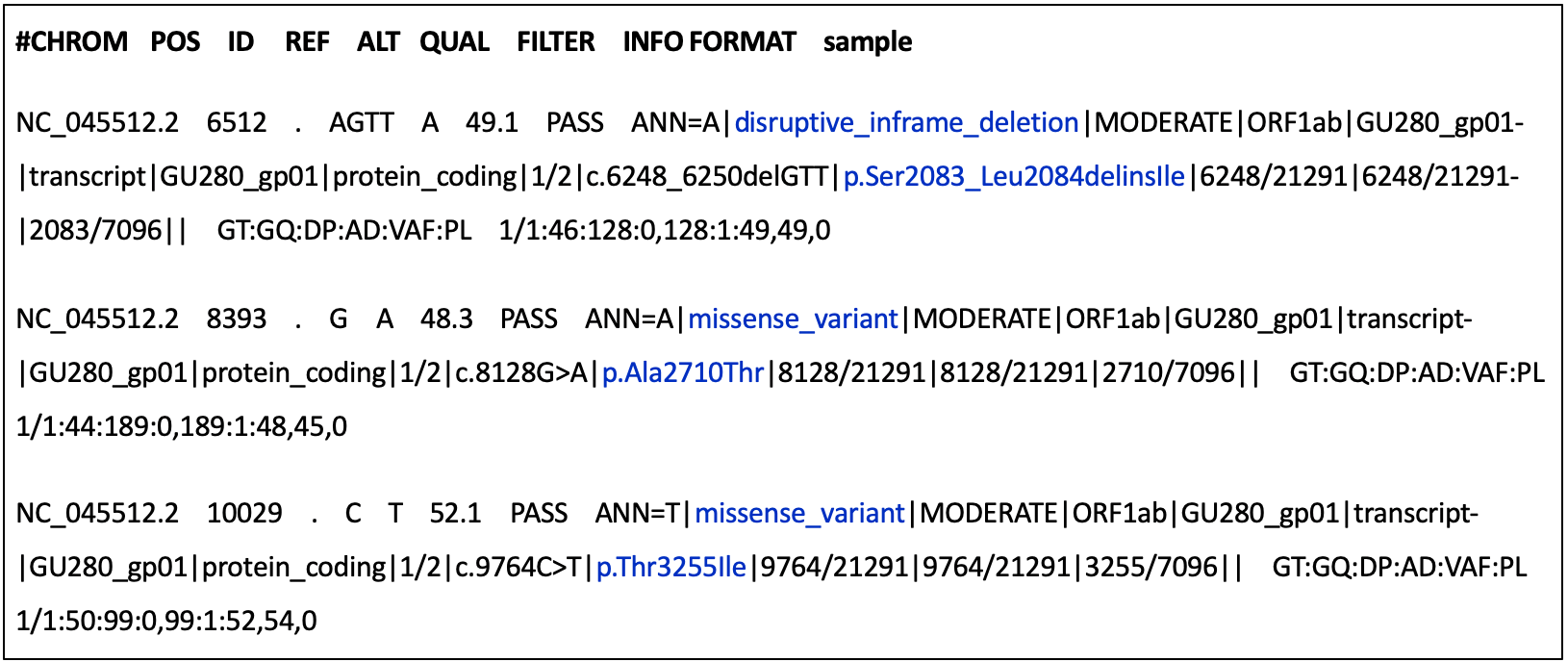
 Identifying candidate variant sites > Ascertaining reference and alternate alleles > Assigning sample genotypes i.e. determining whether alleles are homozygous or heterozygous > (For SARS-CoV-2, all variant and reference alleles should be homozygous (i.e. single-stranded genome) > Variant quality control > Output = VCF file”>
Identifying candidate variant sites > Ascertaining reference and alternate alleles > Assigning sample genotypes i.e. determining whether alleles are homozygous or heterozygous > (For SARS-CoV-2, all variant and reference alleles should be homozygous (i.e. single-stranded genome) > Variant quality control > Output = VCF file”>