Home / Nature & Environment / Chemistry / Identifying Food Fraud / Infrared spectroscopy: an overview

Reach your personal and professional goals
Unlock access to hundreds of expert online courses and degrees from top universities and educators to gain accredited qualifications and professional CV-building certificates.
Join over 18 million learners to launch, switch or build upon your career, all at your own pace, across a wide range of topic areas.


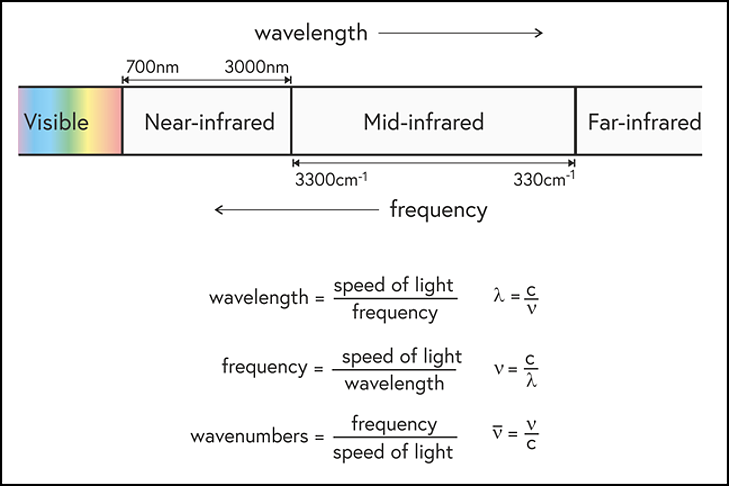 The visible and infrared parts of the electromagnetic spectrum, showing the usual definitions of the near-infrared and mid-infrared wavelength (or frequency) ranges. Also shown are the relationships between wavelength, frequency and wavenumbers
The visible and infrared parts of the electromagnetic spectrum, showing the usual definitions of the near-infrared and mid-infrared wavelength (or frequency) ranges. Also shown are the relationships between wavelength, frequency and wavenumbers
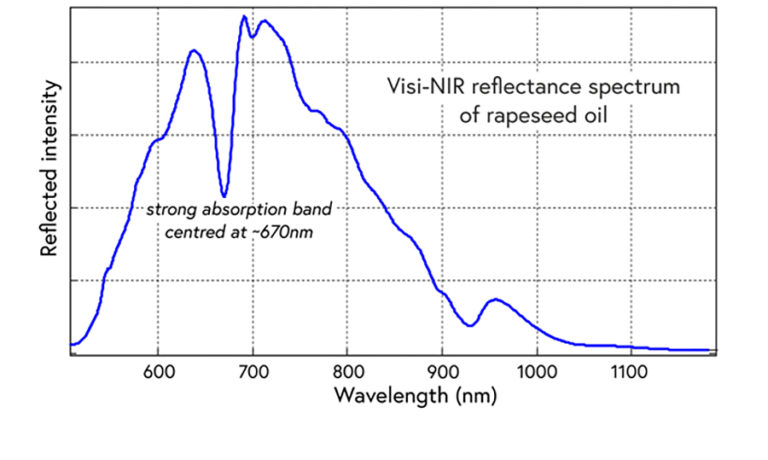 This is a typical visible/near-infrared reflectance spectrum of an edible oil. The strongest feature is an absorption band at around 670nm (approximately the boundary of the visible and NIR regions).
This is a typical visible/near-infrared reflectance spectrum of an edible oil. The strongest feature is an absorption band at around 670nm (approximately the boundary of the visible and NIR regions).
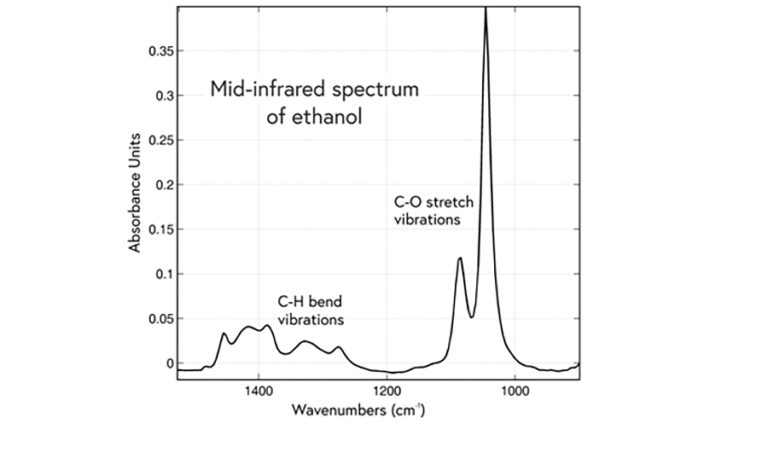 A typical mid-infrared spectrum, of ethanol, showing the “fingerprint” region where the strongest absorption bands occur. Note that a convention in the mid-infrared is for the x-axis scale to be reversed, i.e. with values decreasing from left to right.
A typical mid-infrared spectrum, of ethanol, showing the “fingerprint” region where the strongest absorption bands occur. Note that a convention in the mid-infrared is for the x-axis scale to be reversed, i.e. with values decreasing from left to right.






