Home / Nature & Environment / Chemistry / Identifying Food Fraud / Infrared spectrometers: NIR and MIR compared

Reach your personal and professional goals
Unlock access to hundreds of expert online courses and degrees from top universities and educators to gain accredited qualifications and professional CV-building certificates.
Join over 18 million learners to launch, switch or build upon your career, all at your own pace, across a wide range of topic areas.


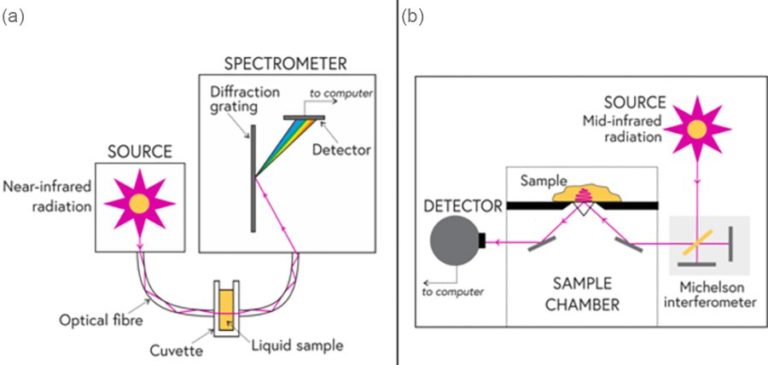 (a) A system for carrying out NIR spectroscopy. An experimental setup for carrying out near-infrared spectroscopy, here using a transmission cell for liquid samples; (b) Inside an FITR spectrometer. A schematic of a Fourier transform infrared (FTIR) spectrometer for carrying out mid-infrared spectroscopy. The sampling method here is called attenuated total reflectance and can be used for examining liquid and solid samples.
(a) A system for carrying out NIR spectroscopy. An experimental setup for carrying out near-infrared spectroscopy, here using a transmission cell for liquid samples; (b) Inside an FITR spectrometer. A schematic of a Fourier transform infrared (FTIR) spectrometer for carrying out mid-infrared spectroscopy. The sampling method here is called attenuated total reflectance and can be used for examining liquid and solid samples.







