Evidence for Fungal Diagnostics
Fungal diagnostic testing ranges from conventional mycological approaches (culture and microscopy) through to the detection of antigens/antibodies and nucleic acids using serological and molecular biomarker assays, respectively. Obviously, in the immunocompromised patient the detection of antibodies will likely be limited by the immune status. In the critically-ill, awaiting the response of humoral immunity in producing antibodies can result in a delay in positivity that restricts the clinical utility of of this approach to patients with chronic/allergic fungal disease.
While culture and microscopy can be applied to a broad range of specimens and detect a wide range of fungi, a reliance on conventional mycological approaches limits testing to confirming infection, the established microbiological strategy. Typically, the sensitivity of culture and microscopy is insufficient (50%), a negative result should not be used to exclude infection, whether this is blood or respiratory culture for the investigation of sepsis and pneumonia, respectively.

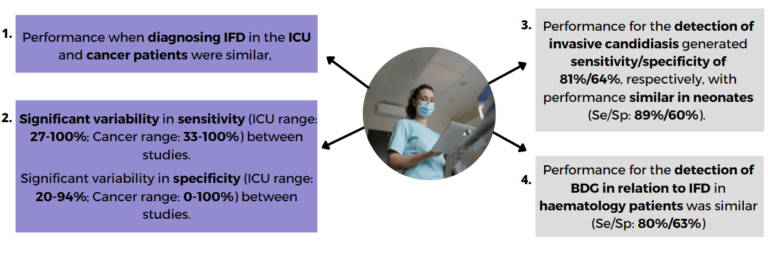
Given the relatively low incidence of invasive fungal disease (IFD <10% in most cohorts), the high pre-test probability of not having infection (>90%) should be exploited by using negative results from highly sensitive test to exclude IFD. The availability of biomarker testing provides sufficient performance to exclude IFD Table 1 but performance can vary depending on sample type, testing strategy, patient population, cause of IFD and the use of antifungal therapy prior to sampling, which has affected both sensitivity and specificity of biomarker testing.
The presence of multiple positive results, whether within a single test type or across multiple different tests, increases the likelihood of IFD. Most tests have been extensively validated in haematology patients compared to critical-care patients, although validation of diagnostics for Pneumocystis pneumonia (PCP) and invasive candidiasis (IC) in the critical-care patient is substantial.
If you require a text version of the above image, this is available as a PDF.
Due to the presence of commensal Candida species and other potential fungal colonisers, BDG testing of respiratory samples is not recommended due to significant false positivity. All positive BDG results should be interpreted in clinical context and with an understanding of the potential specificity issues.
Combining multiple biomarkers enhances confidence when excluding infection, but conversely the presence of multiple positive biomarker results significantly improves specificity for confirming a diagnosis. For the diagnosis of IA various clinical trials have supported the combined used of GM-EIA and Aspergillus PCR, and a meta-analysis of this combined approach generated sensitivity and specificity of 99% and 98%, respectively.
By combining BDG serum testing with PCP PCR of upper respiratory tract samples, a diagnosis of PCP can be confidently achieved or excluded without the need to obtain invasive, deep respiratory samples. The combined strategy for the optimal diagnosis of IC is currently being determined by the A-STOP trial. To read more about this trial please click on the links in the see also section below.
Share this
Fungal Diagnostics in Critically Ill Patients
Fungal Diagnostics in Critically Ill Patients


Reach your personal and professional goals
Unlock access to hundreds of expert online courses and degrees from top universities and educators to gain accredited qualifications and professional CV-building certificates.
Join over 18 million learners to launch, switch or build upon your career, all at your own pace, across a wide range of topic areas.
Register to receive updates
-
Create an account to receive our newsletter, course recommendations and promotions.
Register for free







