Detection of resistance in vector-borne diseases
This step was written by Dr David Weetman, Senior Lecturer, Liverpool School of Tropical Medicine and Dr Michael Coleman, Senior Lecturer in Medical Entomology, Liverpool School of Tropical Medicine.
Why do we need to monitor insecticide resistance?
Insecticidal products are the mainstay of control programmes for most vector-borne diseases. Arthropods often develop resistance to insecticide active ingredients rapidly, and multiple resistance in which the same population is resistant to multiple insecticides is common 1. With resistance in target insects, comes suboptimal control, or even programmatic failure, examples of which are growing especially in malaria control 2. Insecticide Resistance Management (IRM) is a key to sustainable insecticide use 3. However, IRM options such as combination, rotation or mosaic application of insecticides are far more challenging in vector control programmes than in agriculture, primarily owing to a lack of safe and economical insecticides and logistical constrains in large-scale programmes 4. These limitations place particular emphasis on effective warning systems for oncoming insecticide resistance in vector control. Detection of insecticide resistance can occur at a country-wide scale via a strategically chosen set of sentinel sites yielding spatial and temporal data on variation, and/or more focally to investigate changes in susceptibility which may result from application of insecticidal control tools in trials or programmes. Such information is crucial to identify, as early as possible, when changes to a programme may be required to prevent escalation of resistance.
How should we define the resistance we are aiming to detect?
It is first important to identify what type or level of insecticide resistance is of primary concern in a monitoring scenario. There are two main contrasting definitions. The first is aimed at identification of resistance at an early stage and defines resistance as (i) an ability to tolerate doses of an insecticide that would kill the majority of individuals in a susceptible population of that species. The second is concerned with a more advanced stage in which resistance is likely to be well established and is defined as (ii) a heritable population characteristic that results in the repeated failure of an insecticide product to control as intended, when applied correctly 4. Ideally, with entirely effective insecticide resistance management, free of insecticide and economical limitations and logistical constraints, definition (ii) would become redundant, but at present typically both are important for vector resistance monitoring programmes.
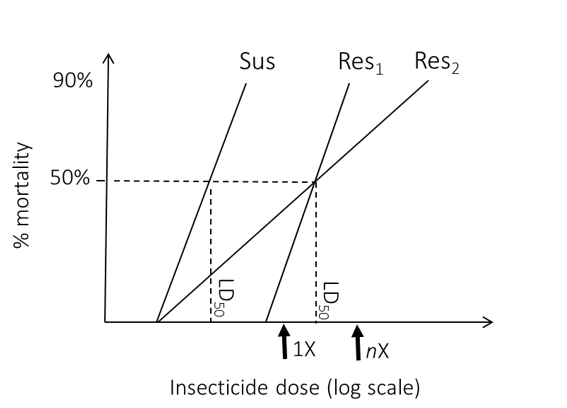
Figure 1: Hypothetical example of bioassay results from a dose-response experiment involving a susceptible (Sus) and two (Res) test populations. Both Res populations show resistance with much higher LD50 than the Sus strain, but Res 2 has a more mixed profile with some individuals highly resistant and others susceptible. This heterogeneity could be captured by application of both standard (1X) and higher level (nX) diagnostic dose bioassays.
What type of approach should be adopted to detect and quantify resistance?
To identify resistance expressed at the phenotypic level, whereby vectors are able to survive specified insecticide doses or exhibit indicative variation between strains or populations, standard methodological approaches are applied.
(a) Discriminating dose bioassays. This methodology is designed to measure the prevalence (i.e. frequency) of resistance in a vector population. Diagnostic doses for several insecticides, which are validated to reliably kill fully susceptible strains are available from WHO 5 and CDC 6 to identify resistant adult mosquitoes following exposure to a specified concentration of insecticide for a specified time. Where a threshold percentage of mosquitoes survive the diagnostic dose, the population is deemed to be resistant. Where possible vector species-specific diagnostic doses should be applied, but doses validated for Anopheles gambiae are often pragmatically applied to culicine mosquitoes 7, and also non-mosquito vectors such as phlebotomine sandflies, for which identification of an appropriate fully susceptible strain for calibration can be challenging. Clearly in such cases diagnoses of resistance or susceptibility must be interpreted with caution.
(b) Dose-response bioassays. This methodology is appropriate to quantify the level and variability of response to insecticide exposure displayed by a population. The tested vectors are exposed to a range of insecticide concentrations for a fixed period of time (or less commonly a fixed dose for a range of exposure times). The combination of a specific concentration x time represents a measure of insecticide dose. From the regression line fitted to data the doses killing specified percentages of the test cohort (i.e. the doses lethal to a given percentage) can be estimated, most commonly at the 50% and 90 or 95% lethal doses (LD50, LD90, LD95). Differences in the slopes and intercepts of the regression line indicate variation in the population profiles for responses to insecticide (Figure 1). This can indicate variation in susceptibility among populations, although identification of resistance intensity per se requires comparison with a susceptible strain at a specific killing dose point (usually the LD50) to estimate a resistance ratio.
(c) Stepwise assessment using multiple discriminating dose bioassays. Dose-response quantification can provide detailed information on resistance prevalence and level within and among populations. However, it is impractical when testing many populations and insecticides against adults because of the large number of test individuals required. Consequently, where diagnostic doses are available, a compromise strategy is now commonly adopted by monitoring teams, whereby if resistance is detected at the diagnostic dose (denoted 1X) progressively higher doses of the insecticide are tested until resistance is no longer detected (e.g. 2X, then 5X, then 10X). This can yield key parts of the information provided by dose-response assays (Figure 1) but using far fewer test individuals.

Figure 2: CDC bottle bioassay in progress: mosquitoes are exposed to insecticide inside coated bottles.
Testing methods for resistance bioassays
There are multiple methods possible for bioassays, but for testing of adult vectors, two are by far the most common. The WHO tube bioassay uses bespoke tube testing kits, within which vectors are exposed to insecticide impregnated paper linings. In the CDC bottle bioassay vectors are introduced into glass bottles, the inner walls of which are coated with insecticide (Figure 2). Detailed protocols have been produced for each method 5, 6, and step-by-step video demonstrations are also available 8, 9. Both methods can be applied for each of the three types of bioassay objective above, and each has practical advantages and disadvantages. Results are expected to be broadly comparable if performed according to protocols and both assays should reliably identify resistance where present but different endpoints make precise comparisons are difficult 5, and it is advisable for monitoring programmes to adopt a single method. An alternative, and more technically challenging, method is topical testing 10, in which an insecticide droplet is apped directly to the dorsal cuticle of an insect. Topical bioassays – using a dose-response approach – are important for testing triatomine vectors 11 and may also occasionally be used to test insecticides against mosquitoes in order to exclude any impact of behavioural variation in the assay 10. Bioassays of larval stage vectors is only common for mosquitoes, which are tested in water to which a serial dilution range of different insecticide concentrations are added in a dose-response protocol to facilitate resistance ratio assessment 12. Irrespective of the method used it is crucial that negative control bioassays without insecticide exposure are run at the same time as the test assays and using a random sample from the same cohort. High mortality (>20%) in controls indicates significant problems with the testing procedure and necessitates repetition of all bioassays, whilst lower levels of control mortality (5-20%) can be accounted for by statistical correction of test data 5. It is good practice to preserve at least a proportion of the tested individuals to allow later checking of species identification, which may require molecular tests for cryptic species, and for molecular identification of resistance mechanisms (below).
Using bioassays to forewarn of insecticidal product failures
Although quantitative links between bioassay data and product control failure remain uncertain, detection of resistance at a higher dose (≥5X) suggests greater potential for failure, i.e. resistance definition (ii) above. Using this warning system additional monitoring procedures of product efficacy against the targeted population are warranted. For example, for bednets and indoor residual sprays, an additional bioassay procedure – the WHO cone test 10 is typically used. Here, adult vectors are enclosed within a small plastic cone attached to the treated net or sprayed wall. If less than 80% of the tested individuals are killed by 24 h after the exposure the insecticide is failing. This might be attributable to insecticide resistance in the local vector population or a substandard impregnation or spray; alternatives which require further investigation using additional cone testing with insecticide susceptible mosquitoes (which should all be killed) or chemical analysis of the insecticide concentration.
Key considerations in the implementation of phenotypic bioassays to monitor resistance
Bioassays are prone to variation, which can affect their interpretation and capacity to detect resistance. In addition to variation between operators, variations in temperature and relative humidity can exert marked effects on bioassay mortality 13, 14 and must be carefully controlled and recorded. Collections may involve sampling larval stages to rear to adults for testing or of gravid females, the offspring of which are reared and tested as adults. In either case collections should constitute a representative sample from the population of interest. Excessive sampling of larvae from few micro-habitats, or the use of offspring derived from a small number of females should be avoided to preclude testing of a highly related cohort, which may provide a biased assessment of population resistance. The WHO and CDC guidelines both specify numbers to be tested (typically a target of 100 per insecticide per dose, plus negative controls 5, 6), but where specific hypotheses of spatial or temporal change are being addressed it is advisable to perform a priori power analyses to determine sample size, for example using freely available software 15. Correct identification of the species studied is crucial, and in the first instance should be performed using standard morphological identification methods. In the case of morphologically cryptic species, supplementary molecular testing will be required 16 at least on a portion of the sample large enough to permit meaningful estimation of the frequency or intensity of resistance in each species present. Without this, spatial or temporal differences in results may arise from a change in relative species abundance rather than resistance per se, which may have different underlying cause.
Insecticide resistance mechanisms
Phenotypic bioassays, and in particular diagnostic dose bioassays are only capable of detecting resistance once it has arisen, and can be relatively insensitive for detection of potentially subtle but potentially important changes in resistance 4. Moreover, whilst phenotypic assays can indicate multiple resistance, only by identification of mechanisms can we understand cross-resistance, in which the same mechanism produces resistance to different insecticides. In addition, some insecticidal control tools are designed to overcome resistance by blocking particular resistance mechanisms. An example of major current importance for malaria control are bednets co-treated with both a pyrethroid insecticide and a synergist molecule piperonyl butoxide (PBO) 17, which inhibits cytochrome P450s, the most important family of enzymes involved in pyrethroid metabolism in insects 18. There are several ways to gain insight into resistance mechanisms operating in a targeted population, but the most practical are synergist bioassays, biochemical tests that assess variation in enzyme activity, and gene- or mutation- specific molecular assays. Though PBO is the most commonly used, synergists are available that block the activity of different enzyme families which may be involved in resistance. If a bioassay performed after pre-exposure to the synergist displays significantly higher mortality than the standard bioassay (without pre-exposure), action of the synergist is demonstrated providing important additional information to inform decisions on implementation of control tools incorporating the synergist molecule 19. Modes of action of synergists can extend beyond blocking the enzyme group targeted 20, sometimes making interpretation of involvement of specific mechanisms ambiguous. However, application is straightforward according to standard bioassay protocols, though the sample size required for testing is doubled 5. Biochemical assays compare the activity level of groups of enzymes among populations and/or with those of a susceptible strain 16. They require a cold chain for sample preservation and lack sensitivity and specificity and links to resistance phenotypes, but in the absence of knowledge of specific resistance mechanisms may provide useful generic information on the presence of types of resistance mechanism.
Molecular surveillance of resistance
If specific resistance mechanisms have been identified, molecular assays can be applied which target either DNA polymorphisms or variation in gene expression. Molecular assays targeting DNA polymorphisms in the target site of insecticides, samples for which do not require such careful handling as those for gene expression, provide opportunities both for identification of important mechanisms and as sensitive partial resistance diagnostics at an operational scale 21, once well calibrated 14, 22. Many such assays are available for diverse vectors including multiple mosquito species 7, 23, sand flies 24 and triatomines 25. An increasing number of DNA assays are also becoming available for other resistance caused by metabolic mechanisms in Anopheles spp. 14, 26, but often these will still require preservation of samples in an RNA fixative to facilitate quantitative studies of the expression levels of key resistance-associated genes 23. A major limitation is the need for species, and often population-specific discovery of resistance linked genes and polymorphisms, although advances in genomic sequence data availability are facilitating these pipelines 27. A second challenge is that resistance mechanisms can be complex and evolve over time, meaning that the diagnostic value of resistance markers must be reassessed periodically and new markers added 28.
Prospectus and challenges
Fortunately, more insecticides are now becoming available for vector control; a trend which should continue over the next decade, allowing far greater opportunities for IRM. Detection of early stage resistance to each is a crucial objective. However, with different modes and durations of action and dependencies on conditions entirely new assay methodologies may need to be developed in order to implement the approaches to detect and quantify resistance. Similarly, whilst molecular surveillance is likely to grow in application, allowing expansion of spatial and temporal screening programmes for resistance expansion, new insecticides bring the challenge of unknown possible resistance mechanisms, which will require novel discovery programmes. Even with the advantage of a greater arsenal of insecticides, given the extreme adaptability shown by insects to resist insecticides 1 we would be wise to avoid complacency and persist with vigilance for upcoming resistance.
Share this
The Global Challenge of Vector Borne Diseases and How to Control Them

The Global Challenge of Vector Borne Diseases and How to Control Them


Reach your personal and professional goals
Unlock access to hundreds of expert online courses and degrees from top universities and educators to gain accredited qualifications and professional CV-building certificates.
Join over 18 million learners to launch, switch or build upon your career, all at your own pace, across a wide range of topic areas.
Register to receive updates
-
Create an account to receive our newsletter, course recommendations and promotions.
Register for free







